

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caspase-2 (Homo sapiens (Human)) | BDBM50160957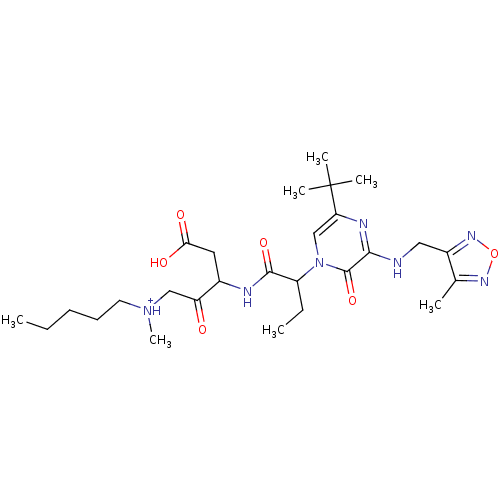 (CHEMBL179503 | [3-(2-{5-tert-Butyl-3-[(4-methyl-fu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration against casp-2 in neuronal precursor (NT2) cells | Bioorg Med Chem Lett 15: 1173-80 (2005) Article DOI: 10.1016/j.bmcl.2004.12.006 BindingDB Entry DOI: 10.7270/Q2D50MGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50160974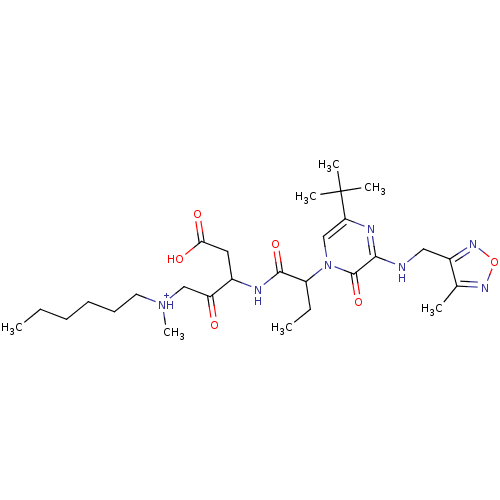 (CHEMBL366927 | [3-(2-{5-tert-Butyl-3-[(4-methyl-fu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Inhibitory concentration against casp-2 in neuronal precursor (NT2) cells | Bioorg Med Chem Lett 15: 1173-80 (2005) Article DOI: 10.1016/j.bmcl.2004.12.006 BindingDB Entry DOI: 10.7270/Q2D50MGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355101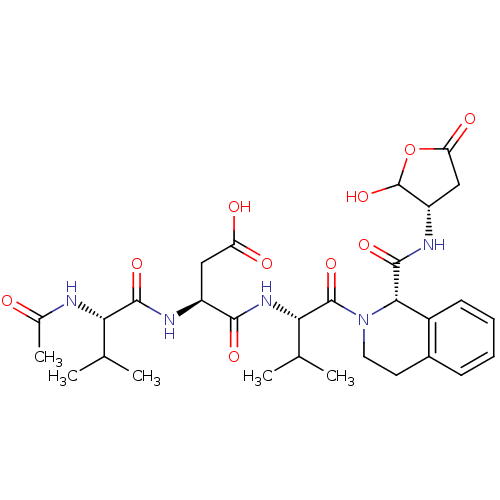 (CHEMBL1835324) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355111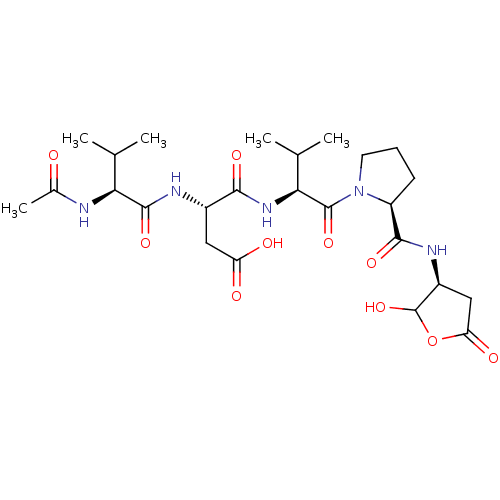 (CHEMBL1835210) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355110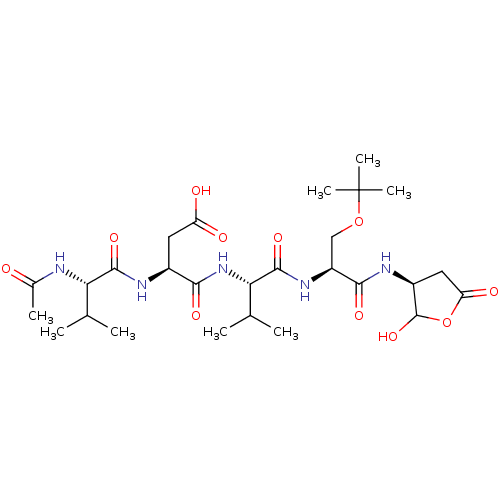 (CHEMBL1835208) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355109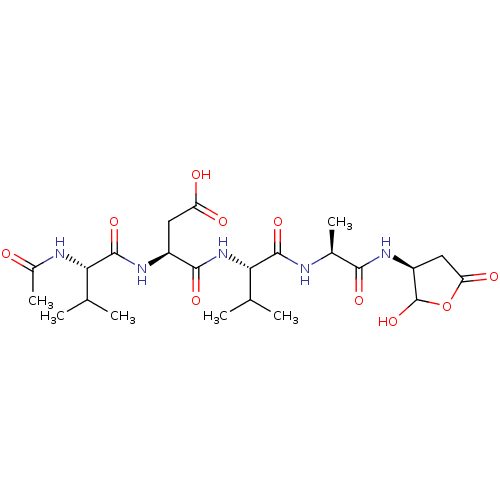 (CHEMBL1835209) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355094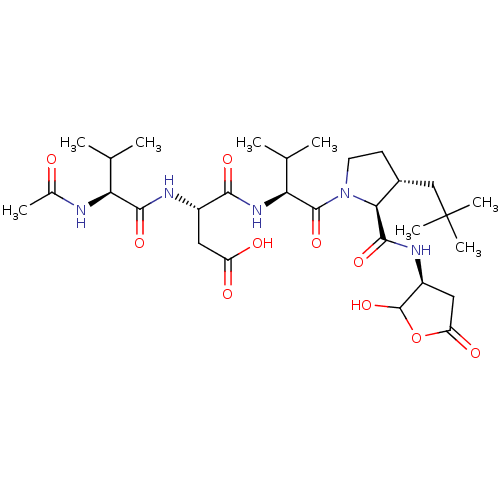 (CHEMBL1835317) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355103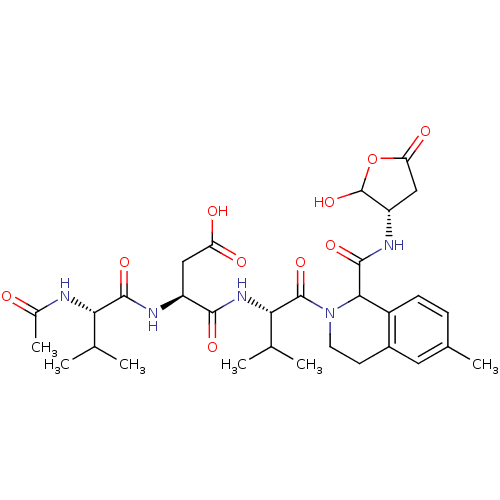 (CHEMBL1835399) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355102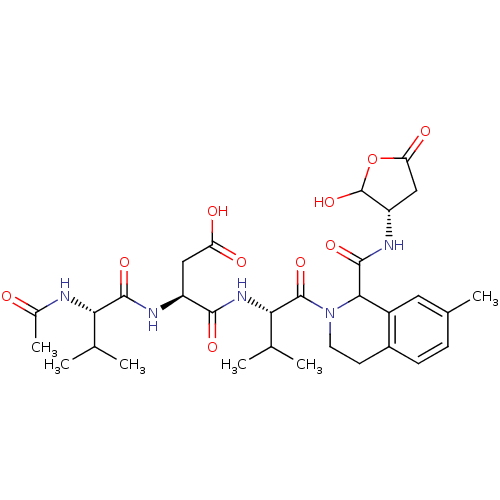 (CHEMBL1835325) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355099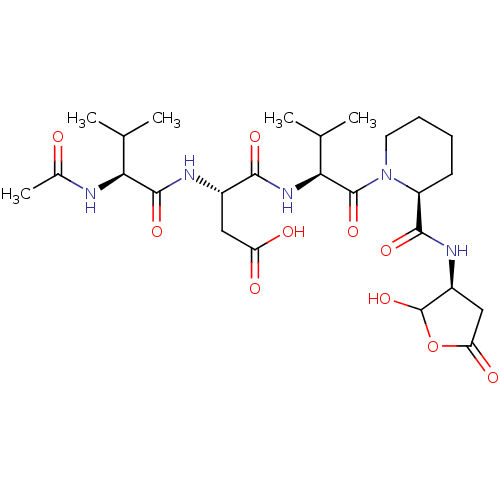 (CHEMBL1835322) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355112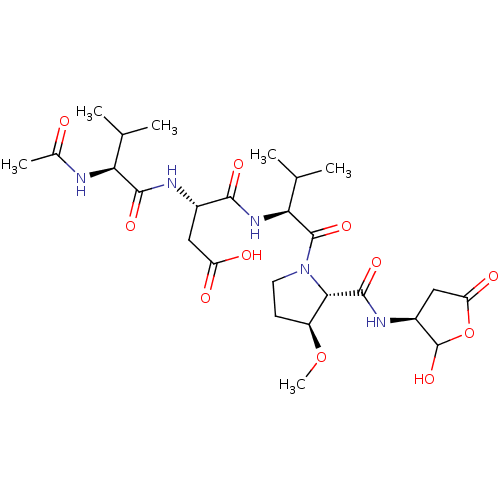 (CHEMBL1835211) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355097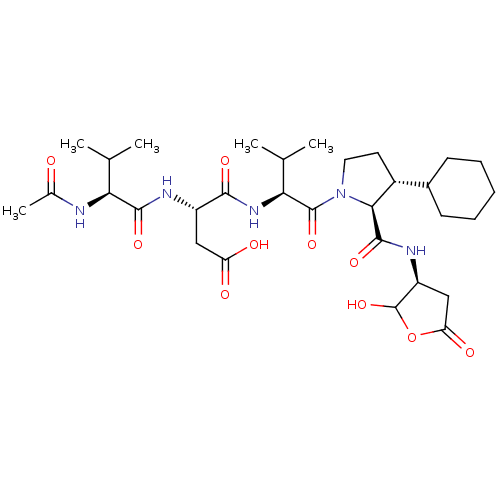 (CHEMBL1835320) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355098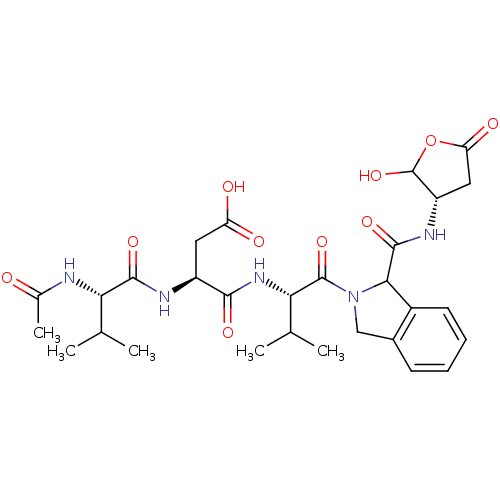 (CHEMBL1835321) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355107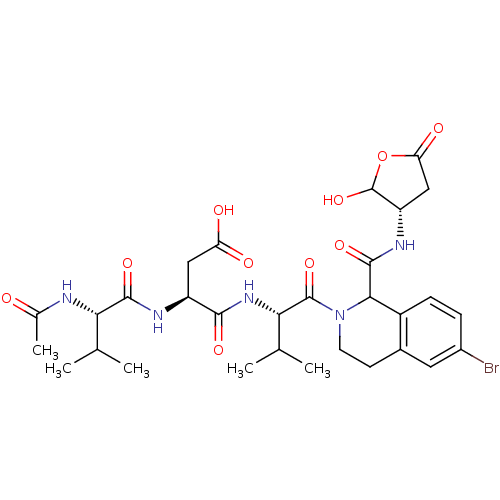 (CHEMBL1835403) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 163 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355093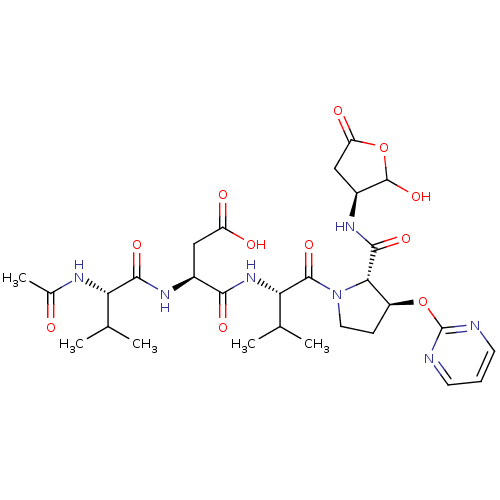 (CHEMBL1835316) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 219 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM10284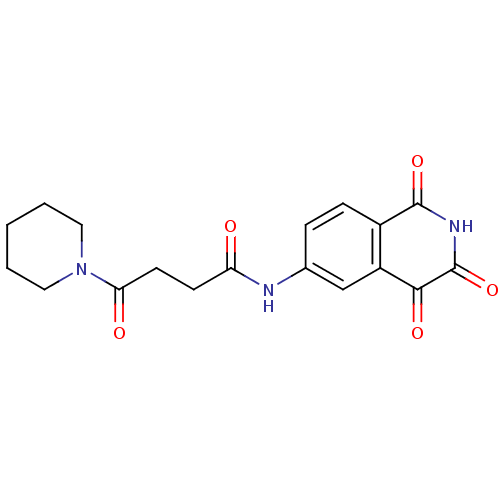 (4-Oxo-4-piperidin-1-yl-N-(1,3,4-trioxo-1,2,3,4-tet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 231 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institutes for Biological Sciences | Assay Description The rate of chromogenic substrate hydrolysis was monitored by the change of absorbance at 405 nm for 3 min. Compounds were tested in duplicate. The I... | J Med Chem 49: 1613-23 (2006) Article DOI: 10.1021/jm050896o BindingDB Entry DOI: 10.7270/Q23N21MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM10264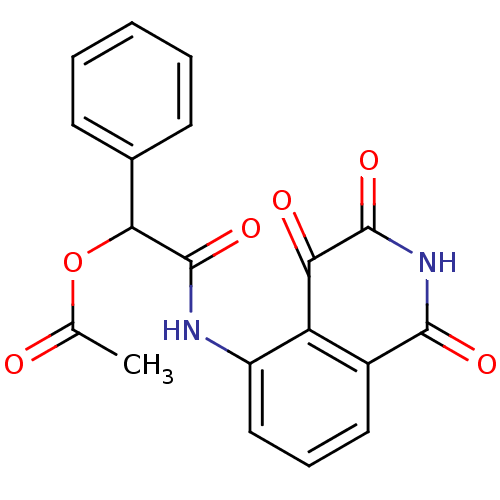 (2-oxo-1-phenyl-2-[(1,3,4-trioxo-1,2,3,4-tetrahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 233 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institutes for Biological Sciences | Assay Description The rate of chromogenic substrate hydrolysis was monitored by the change of absorbance at 405 nm for 3 min. Compounds were tested in duplicate. The I... | J Med Chem 49: 1613-23 (2006) Article DOI: 10.1021/jm050896o BindingDB Entry DOI: 10.7270/Q23N21MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355089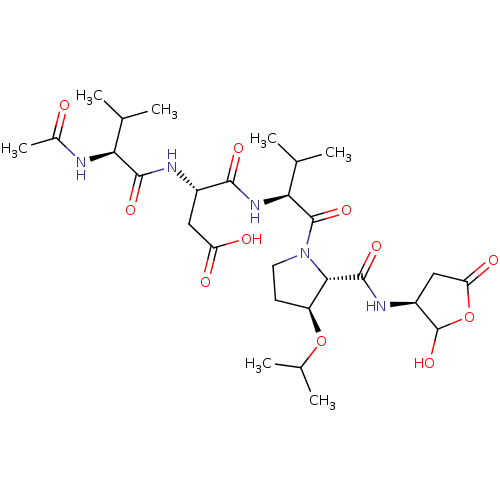 (CHEMBL1835212) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 246 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355090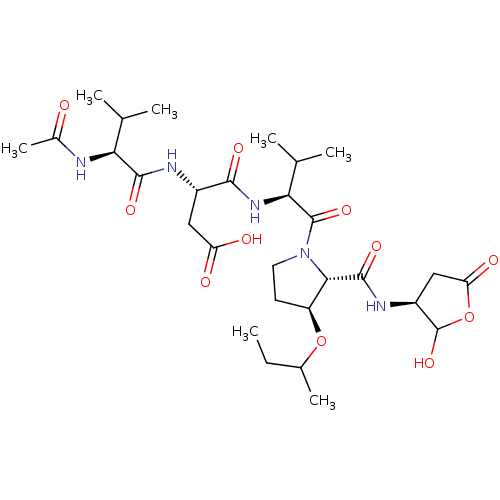 (CHEMBL1835313) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 413 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355095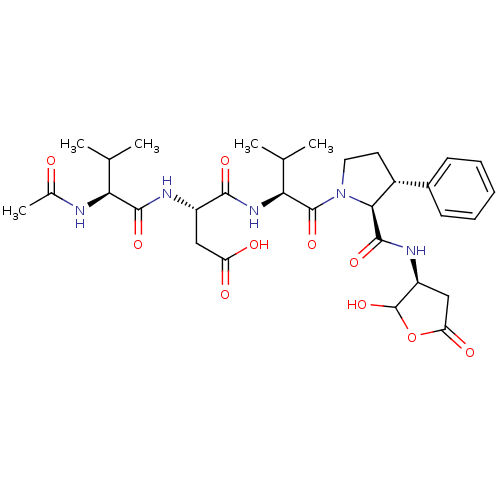 (CHEMBL1835318) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 441 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355091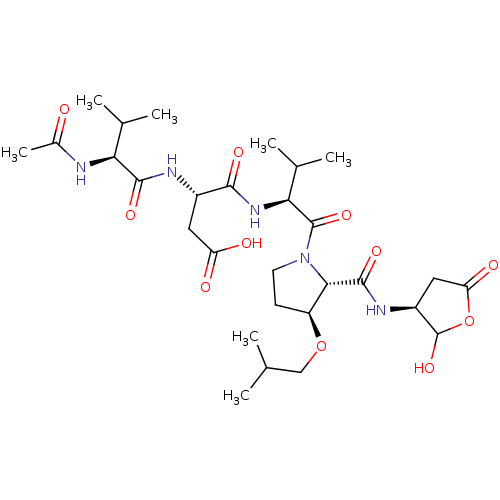 (CHEMBL1835314) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 453 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355106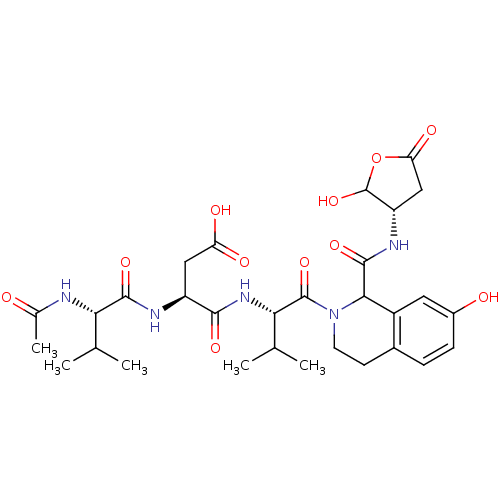 (CHEMBL1835402) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 459 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM10278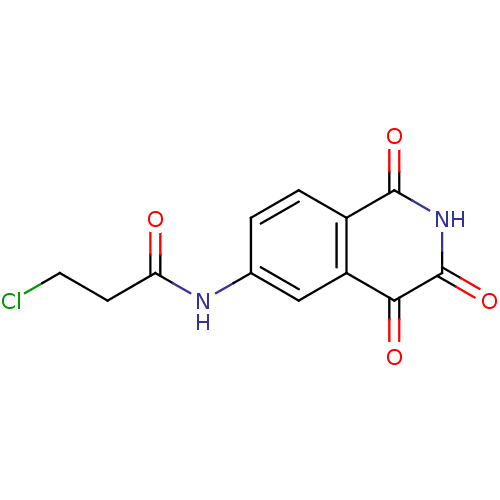 (3-Chloro-N-(1,2,3,4-tetrahydro-1,3,4-trioxoisoquin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institutes for Biological Sciences | Assay Description The rate of chromogenic substrate hydrolysis was monitored by the change of absorbance at 405 nm for 3 min. Compounds were tested in duplicate. The I... | J Med Chem 49: 1613-23 (2006) Article DOI: 10.1021/jm050896o BindingDB Entry DOI: 10.7270/Q23N21MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM10287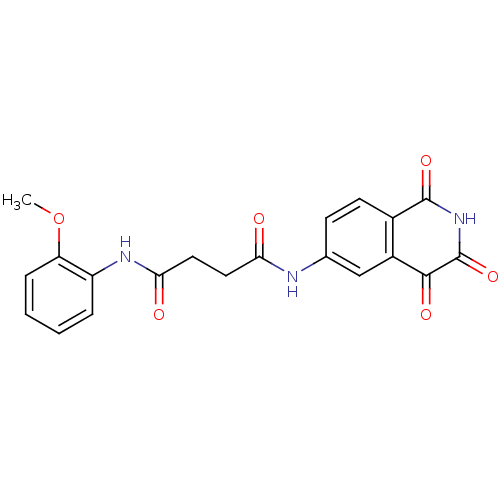 (Isoquinoline-1,3,4-trione 13f | N-(2-Methoxy-pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 537 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institutes for Biological Sciences | Assay Description The rate of chromogenic substrate hydrolysis was monitored by the change of absorbance at 405 nm for 3 min. Compounds were tested in duplicate. The I... | J Med Chem 49: 1613-23 (2006) Article DOI: 10.1021/jm050896o BindingDB Entry DOI: 10.7270/Q23N21MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355108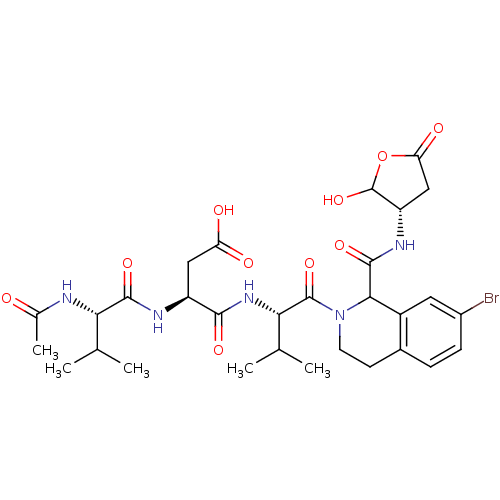 (CHEMBL1835404) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 573 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355111 (CHEMBL1835210) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 635 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of human Myc-DDK tagged caspase-2 expressed in HEK293 T17 cells using Ac-VDVAD-AMC coumarin-120 as substrate pre-incubated for 2 hrs measu... | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355092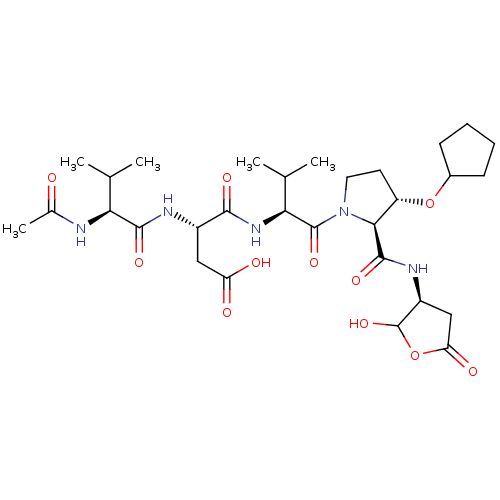 (CHEMBL1835315) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 772 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355098 (CHEMBL1835321) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 781 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of human Myc-DDK tagged caspase-2 expressed in HEK293 T17 cells using Ac-VDVAD-AMC coumarin-120 as substrate pre-incubated for 2 hrs measu... | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM10280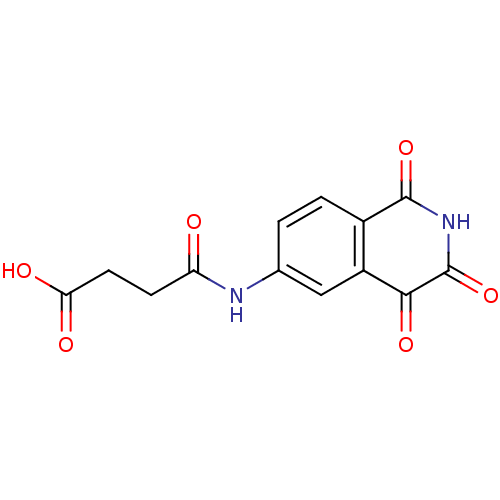 (3-[(1,3,4-trioxo-1,2,3,4-tetrahydroisoquinolin-6-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 859 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institutes for Biological Sciences | Assay Description The rate of chromogenic substrate hydrolysis was monitored by the change of absorbance at 405 nm for 3 min. Compounds were tested in duplicate. The I... | J Med Chem 49: 1613-23 (2006) Article DOI: 10.1021/jm050896o BindingDB Entry DOI: 10.7270/Q23N21MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355099 (CHEMBL1835322) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of human Myc-DDK tagged caspase-2 expressed in HEK293 T17 cells using Ac-VDVAD-AMC coumarin-120 as substrate pre-incubated for 2 hrs measu... | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355109 (CHEMBL1835209) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of human Myc-DDK tagged caspase-2 expressed in HEK293 T17 cells using Ac-VDVAD-AMC coumarin-120 as substrate pre-incubated for 2 hrs measu... | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM10247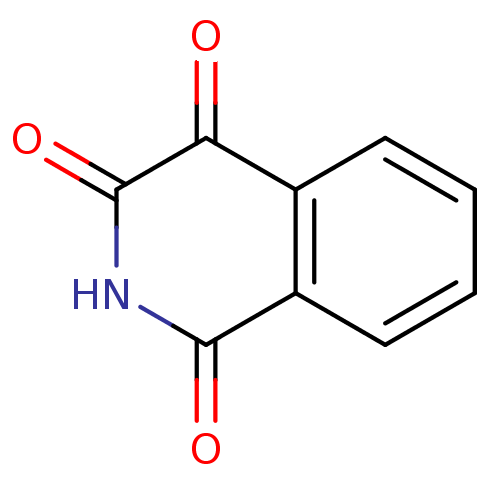 (1,2,3,4-tetrahydroisoquinoline-1,3,4-trione | Isoq...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institutes for Biological Sciences | Assay Description The rate of chromogenic substrate hydrolysis was monitored by the change of absorbance at 405 nm for 3 min. Compounds were tested in duplicate. The I... | J Med Chem 49: 1613-23 (2006) Article DOI: 10.1021/jm050896o BindingDB Entry DOI: 10.7270/Q23N21MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355096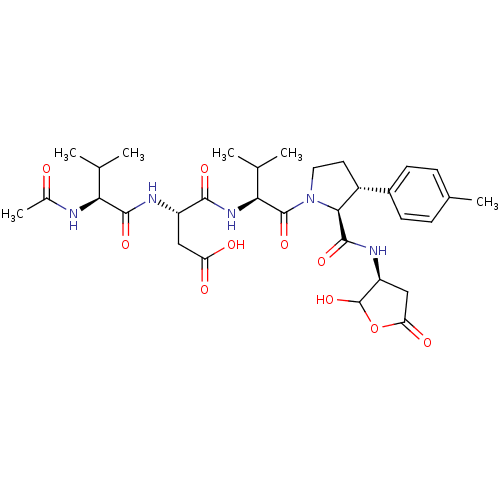 (CHEMBL1835319) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355110 (CHEMBL1835208) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of human Myc-DDK tagged caspase-2 expressed in HEK293 T17 cells using Ac-VDVAD-AMC coumarin-120 as substrate pre-incubated for 2 hrs measu... | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355112 (CHEMBL1835211) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of human Myc-DDK tagged caspase-2 expressed in HEK293 T17 cells using Ac-VDVAD-AMC coumarin-120 as substrate pre-incubated for 2 hrs measu... | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355105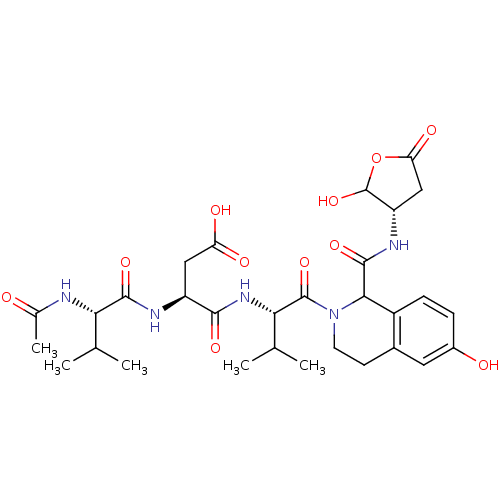 (CHEMBL1835401) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355104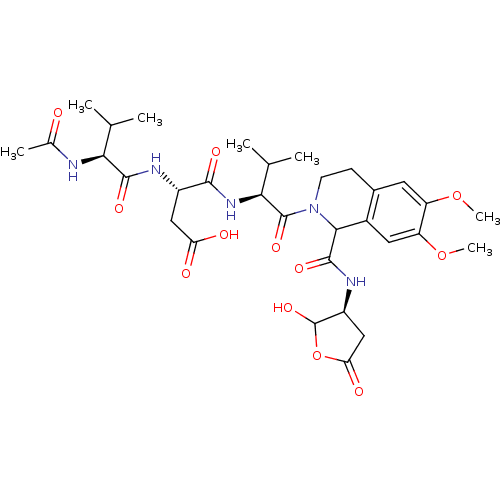 (CHEMBL1835400) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355101 (CHEMBL1835324) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of human Myc-DDK tagged caspase-2 expressed in HEK293 T17 cells using Ac-VDVAD-AMC coumarin-120 as substrate pre-incubated for 2 hrs measu... | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355094 (CHEMBL1835317) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of human Myc-DDK tagged caspase-2 expressed in HEK293 T17 cells using Ac-VDVAD-AMC coumarin-120 as substrate pre-incubated for 2 hrs measu... | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355108 (CHEMBL1835404) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of human Myc-DDK tagged caspase-2 expressed in HEK293 T17 cells using Ac-VDVAD-AMC coumarin-120 as substrate pre-incubated for 2 hrs measu... | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM160786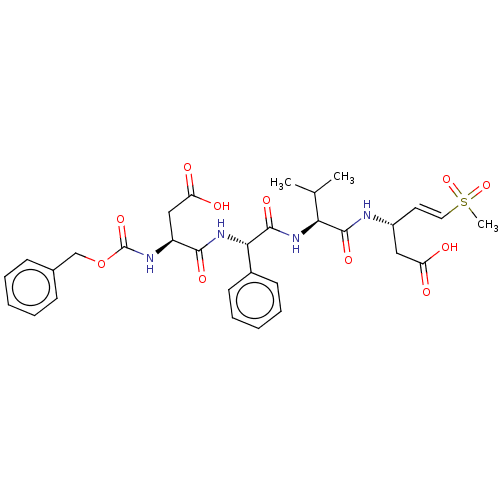 (US10167313, Compound 53 | US9045524, 53) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVAGENESIS FOUNDATION US Patent | Assay Description The inhibition of the activity of Caspase-1 to -10 by four different compounds (#53, #111, #123 & Z-DEVD-FMK used as a prodrug: z-D(OMe)E(OMe)VD(OMe)... | US Patent US9045524 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM160849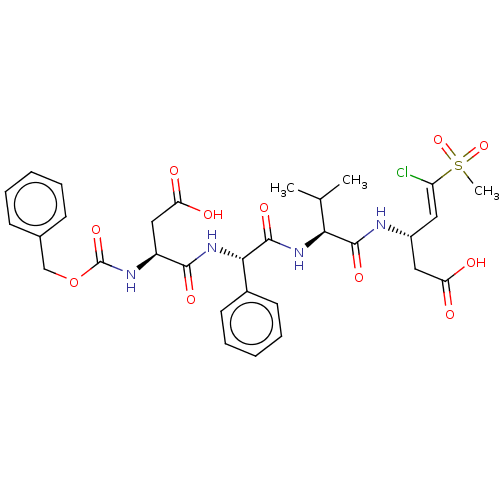 (US9045524, 123) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVAGENESIS FOUNDATION US Patent | Assay Description The inhibition of the activity of Caspase-1 to -10 by four different compounds (#53, #111, #123 & Z-DEVD-FMK used as a prodrug: z-D(OMe)E(OMe)VD(OMe)... | US Patent US9045524 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM160841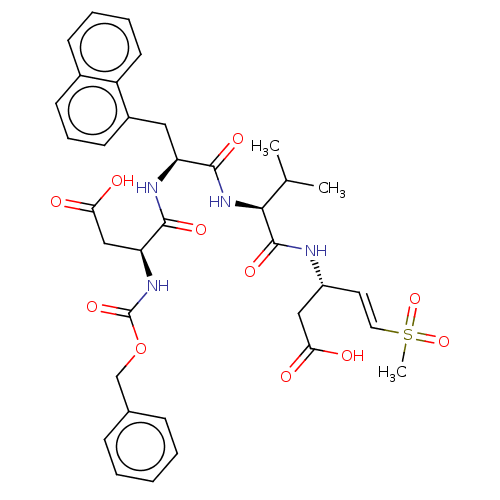 (US9045524, 111) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVAGENESIS FOUNDATION US Patent | Assay Description The inhibition of the activity of Caspase-1 to -10 by four different compounds (#53, #111, #123 & Z-DEVD-FMK used as a prodrug: z-D(OMe)E(OMe)VD(OMe)... | US Patent US9045524 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50302107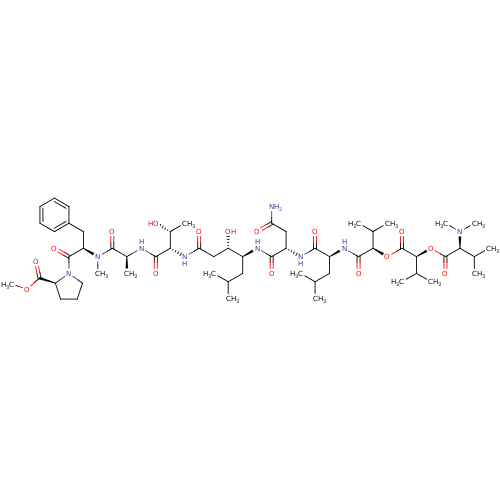 (CHEMBL567893 | Grassystatin A) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of caspase 2 after 10 to 15 mins by fluorescence assay | J Med Chem 52: 5732-47 (2009) Article DOI: 10.1021/jm9009394 BindingDB Entry DOI: 10.7270/Q2BG2PXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355102 (CHEMBL1835325) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of human Myc-DDK tagged caspase-2 expressed in HEK293 T17 cells using Ac-VDVAD-AMC coumarin-120 as substrate pre-incubated for 2 hrs measu... | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM10295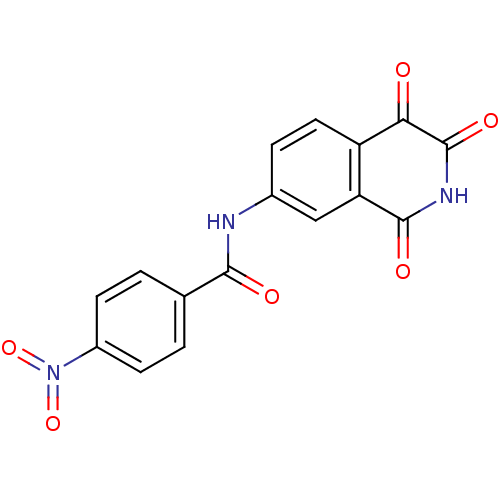 (4-nitro-N-(1,3,4-trioxo-1,2,3,4-tetrahydroisoquino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institutes for Biological Sciences | Assay Description The rate of chromogenic substrate hydrolysis was monitored by the change of absorbance at 405 nm for 3 min. Compounds were tested in duplicate. The I... | J Med Chem 49: 1613-23 (2006) Article DOI: 10.1021/jm050896o BindingDB Entry DOI: 10.7270/Q23N21MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355103 (CHEMBL1835399) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of human Myc-DDK tagged caspase-2 expressed in HEK293 T17 cells using Ac-VDVAD-AMC coumarin-120 as substrate pre-incubated for 2 hrs measu... | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM50355107 (CHEMBL1835403) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc. Curated by ChEMBL | Assay Description Inhibition of human Myc-DDK tagged caspase-2 expressed in HEK293 T17 cells using Ac-VDVAD-AMC coumarin-120 as substrate pre-incubated for 2 hrs measu... | Bioorg Med Chem 19: 5833-51 (2011) Article DOI: 10.1016/j.bmc.2011.08.020 BindingDB Entry DOI: 10.7270/Q20V8D6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PDB UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Caspase 2 | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01063 BindingDB Entry DOI: 10.7270/Q2QJ7MZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-2 (Homo sapiens (Human)) | BDBM480973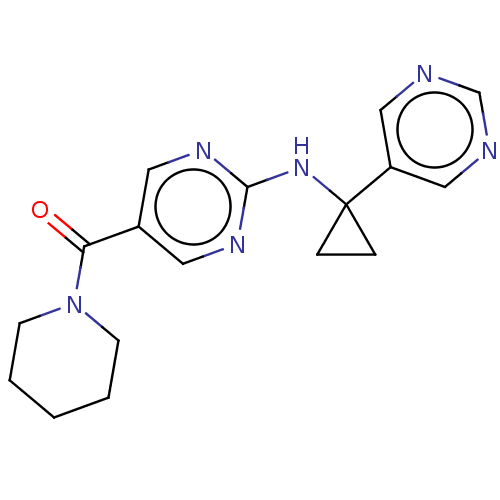 (US10906888, Example 190) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of caspase-2 (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01849 BindingDB Entry DOI: 10.7270/Q2XD15K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 97 total ) | Next | Last >> |