 Found 610 hits Enz. Inhib. hit(s) with Target = 'Chain A, Crystal Structure Of The B1b2 Domains From Human Neuropilin- 1'
Found 610 hits Enz. Inhib. hit(s) with Target = 'Chain A, Crystal Structure Of The B1b2 Domains From Human Neuropilin- 1' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuropilin-1
(Homo sapiens (Human)) | BDBM50453006

(CHEMBL4217624)Show SMILES NCc1ccc(cc1)-c1cc2CCOc2c(c1)S(=O)(=O)Nc1ccsc1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C26H30N6O6S2/c27-14-15-3-5-16(6-4-15)18-12-17-7-10-38-22(17)21(13-18)40(36,37)32-19-8-11-39-23(19)24(33)31-20(25(34)35)2-1-9-30-26(28)29/h3-6,8,11-13,20,32H,1-2,7,9-10,14,27H2,(H,31,33)(H,34,35)(H4,28,29,30)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 609 | n/a | n/a | n/a | n/a | n/a | n/a |
NCE Discovery (Domainex Ltd
Curated by ChEMBL
| Assay Description
Inhibition of biotinylated VEGF-A binding to N-terminal His6-tagged recombinant human NRP1-b1 domain expressed in Escherichia coli Rosetta Gami 2 (DE... |
J Med Chem 61: 4135-4154 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00210
BindingDB Entry DOI: 10.7270/Q2Q81GNK |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50467383

(CHEMBL4279036)Show SMILES [#7]-[#6]-[#6]-[#6](-[#7]-[#6](=O)-[#6](-[#7])-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=[#7])-[#6](-[#8])=O |r| Show InChI InChI=1S/C22H43N11O5/c23-9-8-14(31-17(34)13(24)5-1-2-10-29-21(25)26)19(36)33-12-4-7-16(33)18(35)32-15(20(37)38)6-3-11-30-22(27)28/h13-16H,1-12,23-24H2,(H,31,34)(H,32,35)(H,37,38)(H4,25,26,29)(H4,27,28,30)/t13?,14?,15-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warsaw
Curated by ChEMBL
| Assay Description
Inhibition of biotinylated VEGF165 binding to NRP1 (unknown origin) by ELISA |
Eur J Med Chem 158: 453-462 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.083
BindingDB Entry DOI: 10.7270/Q2B85BT8 |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50453006

(CHEMBL4217624)Show SMILES NCc1ccc(cc1)-c1cc2CCOc2c(c1)S(=O)(=O)Nc1ccsc1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C26H30N6O6S2/c27-14-15-3-5-16(6-4-15)18-12-17-7-10-38-22(17)21(13-18)40(36,37)32-19-8-11-39-23(19)24(33)31-20(25(34)35)2-1-9-30-26(28)29/h3-6,8,11-13,20,32H,1-2,7,9-10,14,27H2,(H,31,33)(H,34,35)(H4,28,29,30)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NCE Discovery (Domainex Ltd
Curated by ChEMBL
| Assay Description
Inhibition of biotinylated VEGF-A binding to human NRP1 expressed in human DU145 cells after 2 hrs |
J Med Chem 61: 4135-4154 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00210
BindingDB Entry DOI: 10.7270/Q2Q81GNK |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50188998

(5-chloro-2-(3-methoxyphenyl)-3-(phenethylamino)ind...)Show SMILES COc1cccc(c1)-c1c(NCCc2ccccc2)n2c(Cl)cccc2c1C#N Show InChI InChI=1S/C24H20ClN3O/c1-29-19-10-5-9-18(15-19)23-20(16-26)21-11-6-12-22(25)28(21)24(23)27-14-13-17-7-3-2-4-8-17/h2-12,15,27H,13-14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie
Curated by ChEMBL
| Assay Description
Inhibition of VEGF165-NRP1 interaction by ELISA |
Bioorg Med Chem Lett 16: 3998-4001 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.014
BindingDB Entry DOI: 10.7270/Q20Z72WD |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50188994
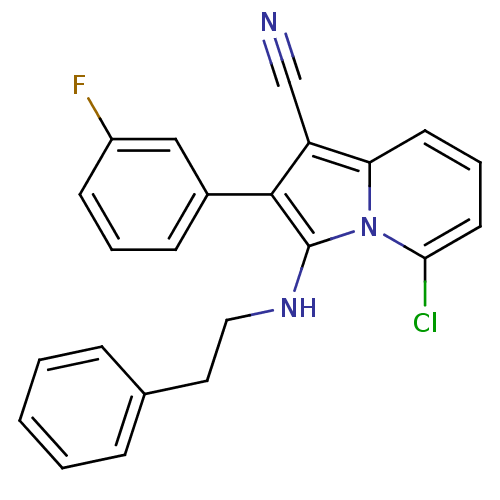
(5-chloro-2-(3-fluorophenyl)-3-(phenethylamino)indo...)Show SMILES Fc1cccc(c1)-c1c(NCCc2ccccc2)n2c(Cl)cccc2c1C#N Show InChI InChI=1S/C23H17ClFN3/c24-21-11-5-10-20-19(15-26)22(17-8-4-9-18(25)14-17)23(28(20)21)27-13-12-16-6-2-1-3-7-16/h1-11,14,27H,12-13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie
Curated by ChEMBL
| Assay Description
Inhibition of VEGF165-NRP1 interaction by ELISA |
Bioorg Med Chem Lett 16: 3998-4001 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.014
BindingDB Entry DOI: 10.7270/Q20Z72WD |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50188970
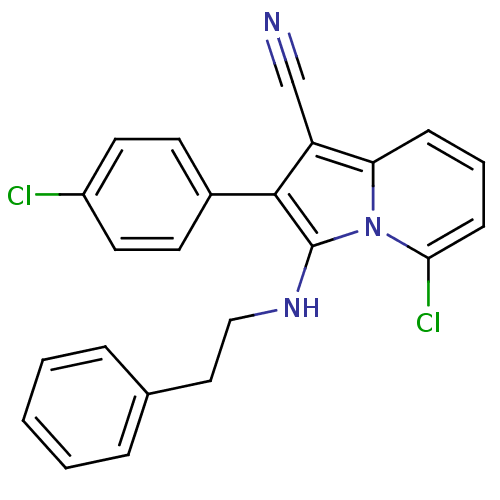
(5-chloro-2-(4-chlorophenyl)-3-(phenethylamino)indo...)Show SMILES Clc1ccc(cc1)-c1c(C#N)c2cccc(Cl)n2c1NCCc1ccccc1 Show InChI InChI=1S/C23H17Cl2N3/c24-18-11-9-17(10-12-18)22-19(15-26)20-7-4-8-21(25)28(20)23(22)27-14-13-16-5-2-1-3-6-16/h1-12,27H,13-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie
Curated by ChEMBL
| Assay Description
Inhibition of VEGF165-NRP1 interaction by ELISA |
Bioorg Med Chem Lett 16: 3998-4001 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.014
BindingDB Entry DOI: 10.7270/Q20Z72WD |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50189004

(5-chloro-2-phenethyl-3-(phenethylamino)indolizine-...)Show SMILES Clc1cccc2c(C#N)c(CCc3ccccc3)c(NCCc3ccccc3)n12 Show InChI InChI=1S/C25H22ClN3/c26-24-13-7-12-23-22(18-27)21(15-14-19-8-3-1-4-9-19)25(29(23)24)28-17-16-20-10-5-2-6-11-20/h1-13,28H,14-17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie
Curated by ChEMBL
| Assay Description
Inhibition of VEGF165-NRP1 interaction by ELISA |
Bioorg Med Chem Lett 16: 3998-4001 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.014
BindingDB Entry DOI: 10.7270/Q20Z72WD |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50188974

(5-chloro-3-(phenethylamino)-2-(pyridin-2-yl)indoli...)Show SMILES Clc1cccc2c(C#N)c(c(NCCc3ccccc3)n12)-c1ccccn1 Show InChI InChI=1S/C22H17ClN4/c23-20-11-6-10-19-17(15-24)21(18-9-4-5-13-25-18)22(27(19)20)26-14-12-16-7-2-1-3-8-16/h1-11,13,26H,12,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie
Curated by ChEMBL
| Assay Description
Inhibition of VEGF165-NRP1 interaction by ELISA |
Bioorg Med Chem Lett 16: 3998-4001 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.014
BindingDB Entry DOI: 10.7270/Q20Z72WD |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50188968
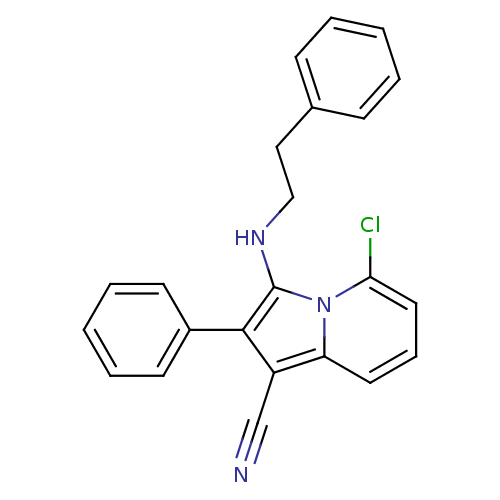
(5-chloro-3-(phenethylamino)-2-phenylindolizine-1-c...)Show SMILES Clc1cccc2c(C#N)c(c(NCCc3ccccc3)n12)-c1ccccc1 Show InChI InChI=1S/C23H18ClN3/c24-21-13-7-12-20-19(16-25)22(18-10-5-2-6-11-18)23(27(20)21)26-15-14-17-8-3-1-4-9-17/h1-13,26H,14-15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie
Curated by ChEMBL
| Assay Description
Inhibition of VEGF165-NRP1 interaction by ELISA |
Bioorg Med Chem Lett 16: 3998-4001 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.014
BindingDB Entry DOI: 10.7270/Q20Z72WD |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50512569

(CHEMBL4476643)Show SMILES NCC[C@@H](NC(=O)[C@@H](N)CCCCNC(=O)NC[C@@H](N)CCCNC(N)=N)C(=O)N1C=CC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r,c:30| Show InChI InChI=1S/C28H54N14O6/c29-11-10-19(24(45)42-15-5-9-21(42)23(44)41-20(25(46)47)8-4-14-37-27(34)35)40-22(43)18(31)7-1-2-12-38-28(48)39-16-17(30)6-3-13-36-26(32)33/h5,15,17-21H,1-4,6-14,16,29-31H2,(H,40,43)(H,41,44)(H,46,47)(H4,32,33,36)(H4,34,35,37)(H2,38,39,48)/t17-,18-,19+,20-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warsaw
Curated by ChEMBL
| Assay Description
Inhibition of biotinylated human VEGF-A165 binding to recombinant human NRP1 receptor after 2 hrs by chemiluminescence analysis relative to control |
Bioorg Med Chem Lett 29: 2493-2497 (2019)
Article DOI: 10.1016/j.bmcl.2019.07.016
BindingDB Entry DOI: 10.7270/Q2C53Q68 |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50512569

(CHEMBL4476643)Show SMILES NCC[C@@H](NC(=O)[C@@H](N)CCCCNC(=O)NC[C@@H](N)CCCNC(N)=N)C(=O)N1C=CC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r,c:30| Show InChI InChI=1S/C28H54N14O6/c29-11-10-19(24(45)42-15-5-9-21(42)23(44)41-20(25(46)47)8-4-14-37-27(34)35)40-22(43)18(31)7-1-2-12-38-28(48)39-16-17(30)6-3-13-36-26(32)33/h5,15,17-21H,1-4,6-14,16,29-31H2,(H,40,43)(H,41,44)(H,46,47)(H4,32,33,36)(H4,34,35,37)(H2,38,39,48)/t17-,18-,19+,20-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warsaw
Curated by ChEMBL
| Assay Description
Inhibition of biotinylated human VEGF-A165 binding to recombinant human NRP1 receptor after 2 hrs by chemiluminescence analysis relative to control |
Bioorg Med Chem Lett 29: 2493-2497 (2019)
Article DOI: 10.1016/j.bmcl.2019.07.016
BindingDB Entry DOI: 10.7270/Q2C53Q68 |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50188990

(5-chloro-2-(2-fluorophenyl)-3-(phenethylamino)indo...)Show SMILES Fc1ccccc1-c1c(NCCc2ccccc2)n2c(Cl)cccc2c1C#N |(25.38,-7.61,;26.16,-8.94,;27.69,-8.93,;28.47,-10.27,;27.7,-11.6,;26.16,-11.6,;25.4,-10.26,;23.86,-10.26,;22.96,-11.51,;23.43,-12.97,;24.94,-13.29,;25.41,-14.76,;26.92,-15.08,;27.39,-16.54,;28.9,-16.86,;29.93,-15.72,;29.45,-14.25,;27.94,-13.93,;21.49,-11.03,;20.16,-11.79,;20.16,-13.33,;18.83,-11.03,;18.83,-9.49,;20.16,-8.71,;21.49,-9.49,;22.96,-9.01,;23.43,-7.55,;23.91,-6.09,)| Show InChI InChI=1S/C23H17ClFN3/c24-21-12-6-11-20-18(15-26)22(17-9-4-5-10-19(17)25)23(28(20)21)27-14-13-16-7-2-1-3-8-16/h1-12,27H,13-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie
Curated by ChEMBL
| Assay Description
Inhibition of VEGF165-NRP1 interaction by ELISA |
Bioorg Med Chem Lett 16: 3998-4001 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.014
BindingDB Entry DOI: 10.7270/Q20Z72WD |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50188979
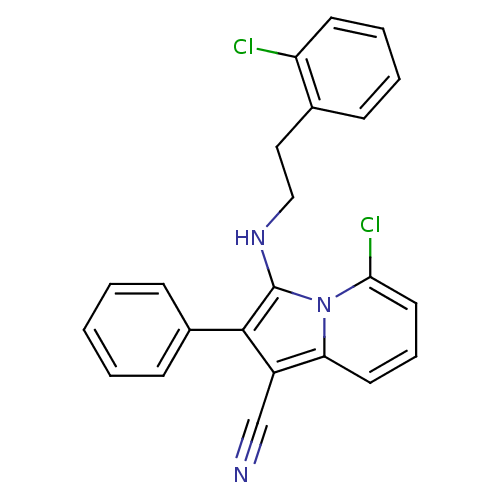
(3-(2-chlorophenethylamino)-5-chloro-2-phenylindoli...)Show SMILES Clc1ccccc1CCNc1c(c(C#N)c2cccc(Cl)n12)-c1ccccc1 Show InChI InChI=1S/C23H17Cl2N3/c24-19-10-5-4-7-16(19)13-14-27-23-22(17-8-2-1-3-9-17)18(15-26)20-11-6-12-21(25)28(20)23/h1-12,27H,13-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie
Curated by ChEMBL
| Assay Description
Inhibition of VEGF165-NRP1 interaction by ELISA |
Bioorg Med Chem Lett 16: 3998-4001 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.014
BindingDB Entry DOI: 10.7270/Q20Z72WD |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50188985

(5-chloro-2-(3-chlorophenyl)-3-(phenethylamino)indo...)Show SMILES Clc1cccc(c1)-c1c(C#N)c2cccc(Cl)n2c1NCCc1ccccc1 Show InChI InChI=1S/C23H17Cl2N3/c24-18-9-4-8-17(14-18)22-19(15-26)20-10-5-11-21(25)28(20)23(22)27-13-12-16-6-2-1-3-7-16/h1-11,14,27H,12-13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie
Curated by ChEMBL
| Assay Description
Inhibition of VEGF165-NRP1 interaction by ELISA |
Bioorg Med Chem Lett 16: 3998-4001 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.014
BindingDB Entry DOI: 10.7270/Q20Z72WD |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50188971

(5-chloro-2-(4-(methylthio)phenyl)-3-(phenethylamin...)Show SMILES CSc1ccc(cc1)-c1c(NCCc2ccccc2)n2c(Cl)cccc2c1C#N Show InChI InChI=1S/C24H20ClN3S/c1-29-19-12-10-18(11-13-19)23-20(16-26)21-8-5-9-22(25)28(21)24(23)27-15-14-17-6-3-2-4-7-17/h2-13,27H,14-15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie
Curated by ChEMBL
| Assay Description
Inhibition of VEGF165-NRP1 interaction by ELISA |
Bioorg Med Chem Lett 16: 3998-4001 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.014
BindingDB Entry DOI: 10.7270/Q20Z72WD |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50188969
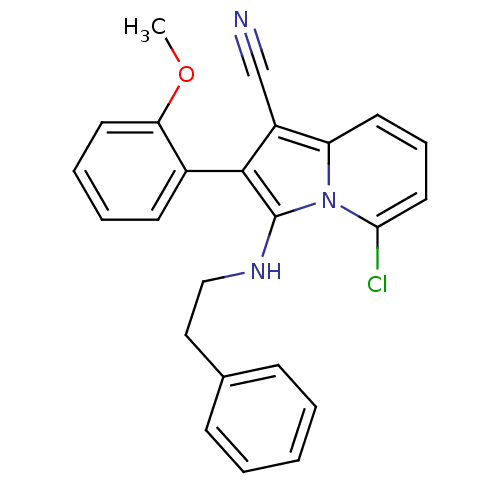
(5-chloro-2-(2-methoxyphenyl)-3-(phenethylamino)ind...)Show SMILES COc1ccccc1-c1c(NCCc2ccccc2)n2c(Cl)cccc2c1C#N |(-1.89,-6.21,;-2.65,-7.54,;-1.88,-8.87,;-.34,-8.87,;.44,-10.2,;-.33,-11.54,;-1.87,-11.54,;-2.63,-10.2,;-4.17,-10.2,;-5.08,-11.45,;-4.6,-12.91,;-3.09,-13.23,;-2.62,-14.69,;-1.11,-15.02,;-.64,-16.48,;.86,-16.8,;1.9,-15.66,;1.41,-14.18,;-.09,-13.87,;-6.54,-10.97,;-7.87,-11.73,;-7.87,-13.27,;-9.2,-10.97,;-9.2,-9.43,;-7.87,-8.65,;-6.54,-9.43,;-5.08,-8.95,;-4.6,-7.49,;-4.12,-6.03,)| Show InChI InChI=1S/C24H20ClN3O/c1-29-21-12-6-5-10-18(21)23-19(16-26)20-11-7-13-22(25)28(20)24(23)27-15-14-17-8-3-2-4-9-17/h2-13,27H,14-15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie
Curated by ChEMBL
| Assay Description
Inhibition of VEGF165-NRP1 interaction by ELISA |
Bioorg Med Chem Lett 16: 3998-4001 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.014
BindingDB Entry DOI: 10.7270/Q20Z72WD |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50313491
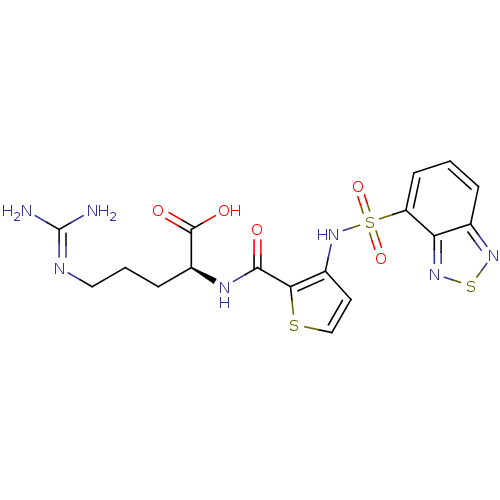
((S)-2-(3-(benzo[c][1,2,5]thiadiazole-4-sulfonamido...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-c1sccc1-[#7]S(=O)(=O)c1cccc2nsnc12)-[#6](-[#8])=O |r| Show InChI InChI=1S/C17H19N7O5S3/c18-17(19)20-7-2-4-11(16(26)27)21-15(25)14-10(6-8-30-14)24-32(28,29)12-5-1-3-9-13(12)23-31-22-9/h1,3,5-6,8,11,24H,2,4,7H2,(H,21,25)(H,26,27)(H4,18,19,20)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd
Curated by ChEMBL
| Assay Description
Displacement of biotinylated-VEGF-A125 from NRP1 b1 domain by cell-free assay |
J Med Chem 53: 2215-26 (2010)
Article DOI: 10.1021/jm901755g
BindingDB Entry DOI: 10.7270/Q2154H62 |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50188967
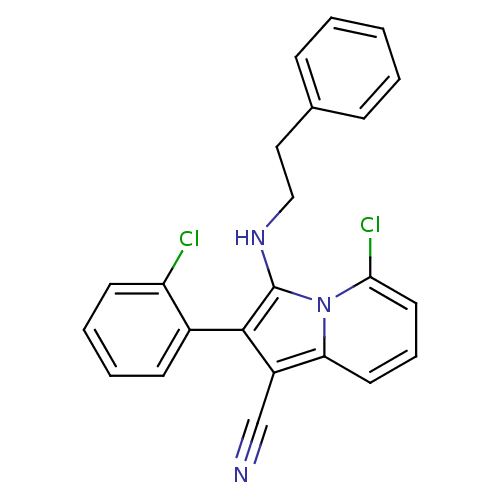
(5-chloro-2-(2-chlorophenyl)-3-(phenethylamino)indo...)Show SMILES Clc1ccccc1-c1c(C#N)c2cccc(Cl)n2c1NCCc1ccccc1 |(10.98,-7.54,;11.76,-8.87,;13.29,-8.87,;14.07,-10.2,;13.3,-11.54,;11.76,-11.54,;11,-10.2,;9.46,-10.2,;8.56,-8.95,;9.03,-7.49,;9.51,-6.03,;7.09,-9.43,;5.76,-8.65,;4.43,-9.43,;4.43,-10.97,;5.76,-11.73,;5.76,-13.27,;7.09,-10.97,;8.56,-11.45,;9.03,-12.91,;10.54,-13.23,;11.02,-14.7,;12.52,-15.02,;12.99,-16.48,;14.5,-16.8,;15.53,-15.66,;15.05,-14.19,;13.54,-13.87,)| Show InChI InChI=1S/C23H17Cl2N3/c24-19-10-5-4-9-17(19)22-18(15-26)20-11-6-12-21(25)28(20)23(22)27-14-13-16-7-2-1-3-8-16/h1-12,27H,13-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie
Curated by ChEMBL
| Assay Description
Inhibition of VEGF165-NRP1 interaction by ELISA |
Bioorg Med Chem Lett 16: 3998-4001 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.014
BindingDB Entry DOI: 10.7270/Q20Z72WD |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50313491

((S)-2-(3-(benzo[c][1,2,5]thiadiazole-4-sulfonamido...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-c1sccc1-[#7]S(=O)(=O)c1cccc2nsnc12)-[#6](-[#8])=O |r| Show InChI InChI=1S/C17H19N7O5S3/c18-17(19)20-7-2-4-11(16(26)27)21-15(25)14-10(6-8-30-14)24-32(28,29)12-5-1-3-9-13(12)23-31-22-9/h1,3,5-6,8,11,24H,2,4,7H2,(H,21,25)(H,26,27)(H4,18,19,20)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd
Curated by ChEMBL
| Assay Description
Displacement of biotinylated-VEGF-A125 from human NRP1 expressed in human DU145 cells |
J Med Chem 53: 2215-26 (2010)
Article DOI: 10.1021/jm901755g
BindingDB Entry DOI: 10.7270/Q2154H62 |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50189000

(3-(4-chlorophenethylamino)-5-chloro-2-phenylindoli...)Show SMILES Clc1ccc(CCNc2c(c(C#N)c3cccc(Cl)n23)-c2ccccc2)cc1 Show InChI InChI=1S/C23H17Cl2N3/c24-18-11-9-16(10-12-18)13-14-27-23-22(17-5-2-1-3-6-17)19(15-26)20-7-4-8-21(25)28(20)23/h1-12,27H,13-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie
Curated by ChEMBL
| Assay Description
Inhibition of VEGF165-NRP1 interaction by ELISA |
Bioorg Med Chem Lett 16: 3998-4001 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.014
BindingDB Entry DOI: 10.7270/Q20Z72WD |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50188982
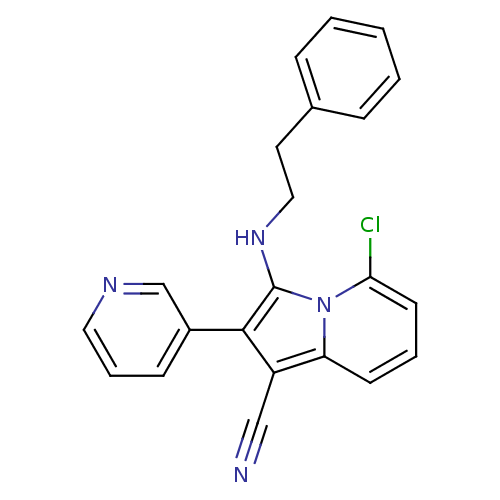
(5-chloro-3-(phenethylamino)-2-(pyridin-3-yl)indoli...)Show SMILES Clc1cccc2c(C#N)c(c(NCCc3ccccc3)n12)-c1cccnc1 Show InChI InChI=1S/C22H17ClN4/c23-20-10-4-9-19-18(14-24)21(17-8-5-12-25-15-17)22(27(19)20)26-13-11-16-6-2-1-3-7-16/h1-10,12,15,26H,11,13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie
Curated by ChEMBL
| Assay Description
Inhibition of VEGF165-NRP1 interaction by ELISA |
Bioorg Med Chem Lett 16: 3998-4001 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.014
BindingDB Entry DOI: 10.7270/Q20Z72WD |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50188999

(5-chloro-2-(4-fluoronaphthalen-1-yl)-3-(phenethyla...)Show SMILES Fc1ccc(-c2c(NCCc3ccccc3)n3c(Cl)cccc3c2C#N)c2ccccc12 |(1.47,1.99,;-.07,1.99,;-.84,.65,;-2.38,.65,;-3.14,1.99,;-4.68,1.99,;-5.58,.74,;-5.11,-.72,;-3.6,-1.04,;-3.13,-2.51,;-1.62,-2.83,;-1.15,-4.29,;.36,-4.61,;1.39,-3.47,;.91,-2,;-.6,-1.68,;-7.05,1.22,;-8.38,.46,;-8.38,-1.08,;-9.71,1.22,;-9.71,2.76,;-8.38,3.54,;-7.05,2.76,;-5.58,3.24,;-5.11,4.7,;-4.63,6.16,;-2.38,3.32,;-3.15,4.64,;-2.39,5.97,;-.85,5.98,;-.08,4.65,;-.84,3.32,)| Show InChI InChI=1S/C27H19ClFN3/c28-25-12-6-11-24-22(17-30)26(21-13-14-23(29)20-10-5-4-9-19(20)21)27(32(24)25)31-16-15-18-7-2-1-3-8-18/h1-14,31H,15-16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie
Curated by ChEMBL
| Assay Description
Inhibition of VEGF165-NRP1 interaction by ELISA |
Bioorg Med Chem Lett 16: 3998-4001 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.014
BindingDB Entry DOI: 10.7270/Q20Z72WD |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50188983

(3-(2-(benzyl(methyl)amino)ethylamino)-5-chloro-2-p...)Show SMILES CN(CCNc1c(c(C#N)c2cccc(Cl)n12)-c1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C25H23ClN4/c1-29(18-19-9-4-2-5-10-19)16-15-28-25-24(20-11-6-3-7-12-20)21(17-27)22-13-8-14-23(26)30(22)25/h2-14,28H,15-16,18H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie
Curated by ChEMBL
| Assay Description
Inhibition of VEGF165-NRP1 interaction by ELISA |
Bioorg Med Chem Lett 16: 3998-4001 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.014
BindingDB Entry DOI: 10.7270/Q20Z72WD |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50313483
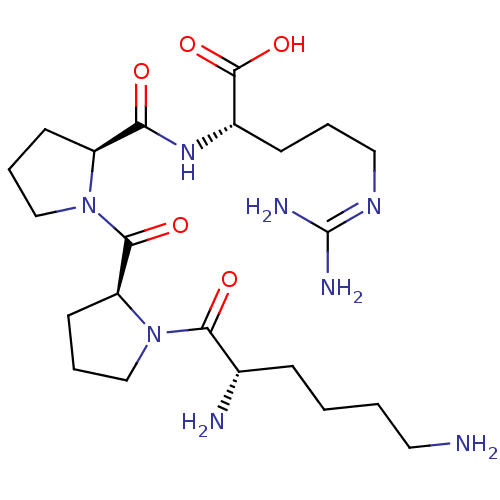
(CHEMBL1091089 | H-KPPR-OH)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#8])=O |r| Show InChI InChI=1S/C22H40N8O5/c23-10-2-1-6-14(24)19(32)30-13-5-9-17(30)20(33)29-12-4-8-16(29)18(31)28-15(21(34)35)7-3-11-27-22(25)26/h14-17H,1-13,23-24H2,(H,28,31)(H,34,35)(H4,25,26,27)/t14-,15-,16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warsaw
Curated by ChEMBL
| Assay Description
Inhibition of biotinylated VEGF165 binding to NRP1 (unknown origin) by ELISA |
Eur J Med Chem 158: 453-462 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.083
BindingDB Entry DOI: 10.7270/Q2B85BT8 |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50188975
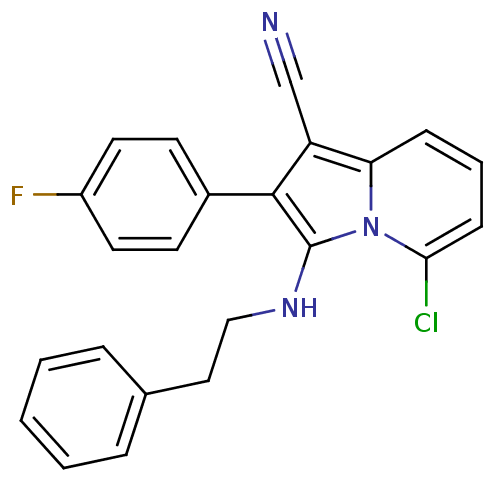
(5-chloro-2-(4-fluorophenyl)-3-(phenethylamino)indo...)Show SMILES Fc1ccc(cc1)-c1c(NCCc2ccccc2)n2c(Cl)cccc2c1C#N Show InChI InChI=1S/C23H17ClFN3/c24-21-8-4-7-20-19(15-26)22(17-9-11-18(25)12-10-17)23(28(20)21)27-14-13-16-5-2-1-3-6-16/h1-12,27H,13-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie
Curated by ChEMBL
| Assay Description
Inhibition of VEGF165-NRP1 interaction by ELISA |
Bioorg Med Chem Lett 16: 3998-4001 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.014
BindingDB Entry DOI: 10.7270/Q20Z72WD |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50189003

(5-chloro-3-(phenethylamino)-2-p-tolylindolizine-1-...)Show SMILES Cc1ccc(cc1)-c1c(NCCc2ccccc2)n2c(Cl)cccc2c1C#N Show InChI InChI=1S/C24H20ClN3/c1-17-10-12-19(13-11-17)23-20(16-26)21-8-5-9-22(25)28(21)24(23)27-15-14-18-6-3-2-4-7-18/h2-13,27H,14-15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie
Curated by ChEMBL
| Assay Description
Inhibition of VEGF165-NRP1 interaction by ELISA |
Bioorg Med Chem Lett 16: 3998-4001 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.014
BindingDB Entry DOI: 10.7270/Q20Z72WD |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50188972

(5-chloro-2-(4-methoxyphenyl)-3-(phenethylamino)ind...)Show SMILES COc1ccc(cc1)-c1c(NCCc2ccccc2)n2c(Cl)cccc2c1C#N Show InChI InChI=1S/C24H20ClN3O/c1-29-19-12-10-18(11-13-19)23-20(16-26)21-8-5-9-22(25)28(21)24(23)27-15-14-17-6-3-2-4-7-17/h2-13,27H,14-15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie
Curated by ChEMBL
| Assay Description
Inhibition of VEGF165-NRP1 interaction by ELISA |
Bioorg Med Chem Lett 16: 3998-4001 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.014
BindingDB Entry DOI: 10.7270/Q20Z72WD |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50512567

(CHEMBL4586661)Show SMILES NCC[C@@H](NC(=O)[C@@H](N)CCCCNC(=O)NC[C@@H](N)CCCNC(N)=N)C(=O)N1[C@@H](CC2CCCCC12)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C32H62N14O6/c33-13-12-22(44-26(47)21(35)9-3-4-14-42-32(52)43-18-20(34)8-5-15-40-30(36)37)28(49)46-24-11-2-1-7-19(24)17-25(46)27(48)45-23(29(50)51)10-6-16-41-31(38)39/h19-25H,1-18,33-35H2,(H,44,47)(H,45,48)(H,50,51)(H4,36,37,40)(H4,38,39,41)(H2,42,43,52)/t19?,20-,21-,22+,23-,24?,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warsaw
Curated by ChEMBL
| Assay Description
Inhibition of biotinylated human VEGF-A165 binding to recombinant human NRP1 receptor after 2 hrs by chemiluminescence analysis relative to control |
Bioorg Med Chem Lett 29: 2493-2497 (2019)
Article DOI: 10.1016/j.bmcl.2019.07.016
BindingDB Entry DOI: 10.7270/Q2C53Q68 |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50512567

(CHEMBL4586661)Show SMILES NCC[C@@H](NC(=O)[C@@H](N)CCCCNC(=O)NC[C@@H](N)CCCNC(N)=N)C(=O)N1[C@@H](CC2CCCCC12)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C32H62N14O6/c33-13-12-22(44-26(47)21(35)9-3-4-14-42-32(52)43-18-20(34)8-5-15-40-30(36)37)28(49)46-24-11-2-1-7-19(24)17-25(46)27(48)45-23(29(50)51)10-6-16-41-31(38)39/h19-25H,1-18,33-35H2,(H,44,47)(H,45,48)(H,50,51)(H4,36,37,40)(H4,38,39,41)(H2,42,43,52)/t19?,20-,21-,22+,23-,24?,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warsaw
Curated by ChEMBL
| Assay Description
Inhibition of biotinylated human VEGF-A165 binding to recombinant human NRP1 receptor after 2 hrs by chemiluminescence analysis relative to control |
Bioorg Med Chem Lett 29: 2493-2497 (2019)
Article DOI: 10.1016/j.bmcl.2019.07.016
BindingDB Entry DOI: 10.7270/Q2C53Q68 |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50188992

(5-chloro-3-(phenethylamino)-2-(pyridin-4-yl)indoli...)Show SMILES Clc1cccc2c(C#N)c(c(NCCc3ccccc3)n12)-c1ccncc1 Show InChI InChI=1S/C22H17ClN4/c23-20-8-4-7-19-18(15-24)21(17-10-12-25-13-11-17)22(27(19)20)26-14-9-16-5-2-1-3-6-16/h1-8,10-13,26H,9,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie
Curated by ChEMBL
| Assay Description
Inhibition of VEGF165-NRP1 interaction by ELISA |
Bioorg Med Chem Lett 16: 3998-4001 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.014
BindingDB Entry DOI: 10.7270/Q20Z72WD |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50313494
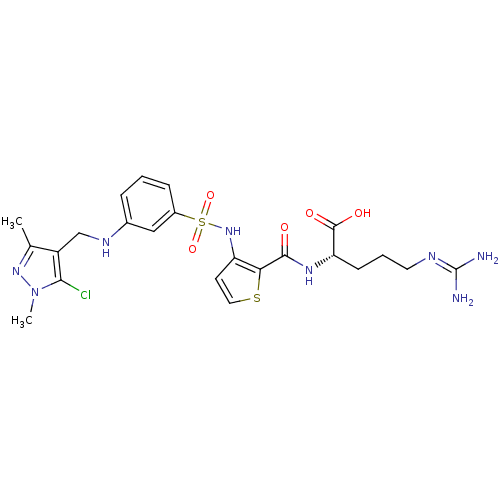
((S)-2-[(3-{3-[(5-Chloro-1,3-dimethyl-1H-pyrazol-4-...)Show SMILES [#6]-c1nn(-[#6])c(Cl)c1-[#6]-[#7]-c1cccc(c1)S(=O)(=O)[#7]-c1ccsc1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#8])=O |r| Show InChI InChI=1S/C23H29ClN8O5S2/c1-13-16(20(24)32(2)30-13)12-28-14-5-3-6-15(11-14)39(36,37)31-17-8-10-38-19(17)21(33)29-18(22(34)35)7-4-9-27-23(25)26/h3,5-6,8,10-11,18,28,31H,4,7,9,12H2,1-2H3,(H,29,33)(H,34,35)(H4,25,26,27)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]VEGF-A from human NRP1 expressed in pig aortic endothelial cells |
J Med Chem 53: 2215-26 (2010)
Article DOI: 10.1021/jm901755g
BindingDB Entry DOI: 10.7270/Q2154H62 |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50188984

(5-chloro-2-(3-mercaptophenyl)-3-(phenethylamino)in...)Show SMILES Sc1cccc(c1)-c1c(NCCc2ccccc2)n2c(Cl)cccc2c1C#N Show InChI InChI=1S/C23H18ClN3S/c24-21-11-5-10-20-19(15-25)22(17-8-4-9-18(28)14-17)23(27(20)21)26-13-12-16-6-2-1-3-7-16/h1-11,14,26,28H,12-13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie
Curated by ChEMBL
| Assay Description
Inhibition of VEGF165-NRP1 interaction by ELISA |
Bioorg Med Chem Lett 16: 3998-4001 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.014
BindingDB Entry DOI: 10.7270/Q20Z72WD |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50188997

(5-chloro-2-(2,5-dimethylphenyl)-3-(phenethylamino)...)Show SMILES Cc1ccc(C)c(c1)-c1c(NCCc2ccccc2)n2c(Cl)cccc2c1C#N |(14.97,-35.83,;14.2,-34.49,;14.97,-33.16,;14.19,-31.82,;12.65,-31.83,;11.88,-30.5,;11.9,-33.15,;12.66,-34.49,;10.36,-33.15,;9.45,-34.4,;9.93,-35.87,;11.44,-36.19,;11.91,-37.65,;13.42,-37.97,;13.89,-39.44,;15.39,-39.76,;16.43,-38.61,;15.94,-37.14,;14.44,-36.83,;7.99,-33.92,;6.66,-34.68,;6.66,-36.22,;5.33,-33.92,;5.33,-32.38,;6.66,-31.6,;7.99,-32.38,;9.45,-31.91,;9.93,-30.44,;10.41,-28.98,)| Show InChI InChI=1S/C25H22ClN3/c1-17-11-12-18(2)20(15-17)24-21(16-27)22-9-6-10-23(26)29(22)25(24)28-14-13-19-7-4-3-5-8-19/h3-12,15,28H,13-14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie
Curated by ChEMBL
| Assay Description
Inhibition of VEGF165-NRP1 interaction by ELISA |
Bioorg Med Chem Lett 16: 3998-4001 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.014
BindingDB Entry DOI: 10.7270/Q20Z72WD |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50313491

((S)-2-(3-(benzo[c][1,2,5]thiadiazole-4-sulfonamido...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-c1sccc1-[#7]S(=O)(=O)c1cccc2nsnc12)-[#6](-[#8])=O |r| Show InChI InChI=1S/C17H19N7O5S3/c18-17(19)20-7-2-4-11(16(26)27)21-15(25)14-10(6-8-30-14)24-32(28,29)12-5-1-3-9-13(12)23-31-22-9/h1,3,5-6,8,11,24H,2,4,7H2,(H,21,25)(H,26,27)(H4,18,19,20)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]VEGF-A from human NRP1 expressed in human A549 cells |
J Med Chem 53: 2215-26 (2010)
Article DOI: 10.1021/jm901755g
BindingDB Entry DOI: 10.7270/Q2154H62 |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50512570

(CHEMBL4549611)Show SMILES N[C@@H](CCCNC(N)=N)CNC(=O)NCCCC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C29H55N13O6/c30-18(7-3-13-36-27(32)33)17-39-29(48)38-12-2-1-8-19(31)24(44)42-16-6-11-22(42)25(45)41-15-5-10-21(41)23(43)40-20(26(46)47)9-4-14-37-28(34)35/h18-22H,1-17,30-31H2,(H,40,43)(H,46,47)(H4,32,33,36)(H4,34,35,37)(H2,38,39,48)/t18-,19-,20-,21-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warsaw
Curated by ChEMBL
| Assay Description
Inhibition of biotinylated human VEGF-A165 binding to recombinant human NRP1 receptor after 2 hrs by chemiluminescence analysis relative to control |
Bioorg Med Chem Lett 29: 2493-2497 (2019)
Article DOI: 10.1016/j.bmcl.2019.07.016
BindingDB Entry DOI: 10.7270/Q2C53Q68 |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50512570

(CHEMBL4549611)Show SMILES N[C@@H](CCCNC(N)=N)CNC(=O)NCCCC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C29H55N13O6/c30-18(7-3-13-36-27(32)33)17-39-29(48)38-12-2-1-8-19(31)24(44)42-16-6-11-22(42)25(45)41-15-5-10-21(41)23(43)40-20(26(46)47)9-4-14-37-28(34)35/h18-22H,1-17,30-31H2,(H,40,43)(H,46,47)(H4,32,33,36)(H4,34,35,37)(H2,38,39,48)/t18-,19-,20-,21-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warsaw
Curated by ChEMBL
| Assay Description
Inhibition of biotinylated human VEGF-A165 binding to recombinant human NRP1 receptor after 2 hrs by chemiluminescence analysis relative to control |
Bioorg Med Chem Lett 29: 2493-2497 (2019)
Article DOI: 10.1016/j.bmcl.2019.07.016
BindingDB Entry DOI: 10.7270/Q2C53Q68 |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50313491

((S)-2-(3-(benzo[c][1,2,5]thiadiazole-4-sulfonamido...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-c1sccc1-[#7]S(=O)(=O)c1cccc2nsnc12)-[#6](-[#8])=O |r| Show InChI InChI=1S/C17H19N7O5S3/c18-17(19)20-7-2-4-11(16(26)27)21-15(25)14-10(6-8-30-14)24-32(28,29)12-5-1-3-9-13(12)23-31-22-9/h1,3,5-6,8,11,24H,2,4,7H2,(H,21,25)(H,26,27)(H4,18,19,20)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]VEGF-A from human NRP1 expressed in pig aortic endothelial cells |
J Med Chem 53: 2215-26 (2010)
Article DOI: 10.1021/jm901755g
BindingDB Entry DOI: 10.7270/Q2154H62 |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50188996
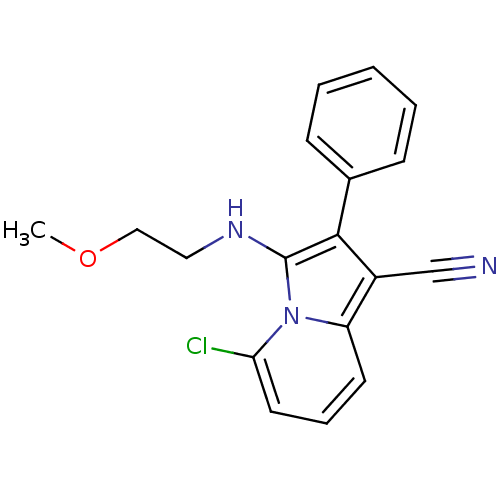
(5-chloro-3-(2-methoxyethylamino)-2-phenylindolizin...)Show InChI InChI=1S/C18H16ClN3O/c1-23-11-10-21-18-17(13-6-3-2-4-7-13)14(12-20)15-8-5-9-16(19)22(15)18/h2-9,21H,10-11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie
Curated by ChEMBL
| Assay Description
Inhibition of VEGF165-NRP1 interaction by ELISA |
Bioorg Med Chem Lett 16: 3998-4001 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.014
BindingDB Entry DOI: 10.7270/Q20Z72WD |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50313489
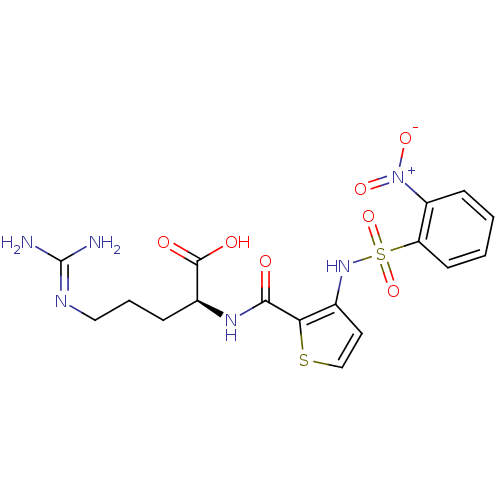
((S)-2-{[3-(2-nitro-benzenesulfonylamino)-thiophene...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-c1sccc1-[#7]S(=O)(=O)c1ccccc1-[#7+](-[#8-])=O)-[#6](-[#8])=O |r| Show InChI InChI=1S/C17H20N6O7S2/c18-17(19)20-8-3-4-11(16(25)26)21-15(24)14-10(7-9-31-14)22-32(29,30)13-6-2-1-5-12(13)23(27)28/h1-2,5-7,9,11,22H,3-4,8H2,(H,21,24)(H,25,26)(H4,18,19,20)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]VEGF-A from human NRP1 expressed in pig aortic endothelial cells |
J Med Chem 53: 2215-26 (2010)
Article DOI: 10.1021/jm901755g
BindingDB Entry DOI: 10.7270/Q2154H62 |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50188986
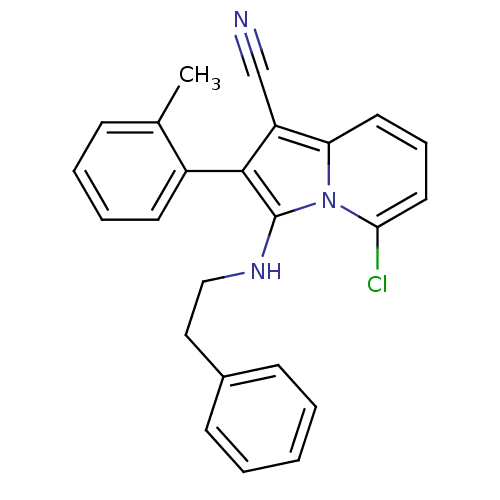
(5-chloro-3-(phenethylamino)-2-o-tolylindolizine-1-...)Show SMILES Cc1ccccc1-c1c(NCCc2ccccc2)n2c(Cl)cccc2c1C#N |(11.82,-18.37,;12.6,-19.7,;14.13,-19.69,;14.91,-21.03,;14.14,-22.36,;12.6,-22.36,;11.84,-21.02,;10.3,-21.02,;9.4,-22.27,;9.87,-23.74,;11.38,-24.06,;11.86,-25.52,;13.36,-25.84,;13.83,-27.31,;15.34,-27.63,;16.37,-26.48,;15.89,-25.01,;14.38,-24.7,;7.93,-21.8,;6.6,-22.56,;6.6,-24.1,;5.27,-21.79,;5.27,-20.25,;6.6,-19.48,;7.93,-20.25,;9.4,-19.78,;9.87,-18.31,;10.35,-16.85,)| Show InChI InChI=1S/C24H20ClN3/c1-17-8-5-6-11-19(17)23-20(16-26)21-12-7-13-22(25)28(21)24(23)27-15-14-18-9-3-2-4-10-18/h2-13,27H,14-15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie
Curated by ChEMBL
| Assay Description
Inhibition of VEGF165-NRP1 interaction by ELISA |
Bioorg Med Chem Lett 16: 3998-4001 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.014
BindingDB Entry DOI: 10.7270/Q20Z72WD |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM94385
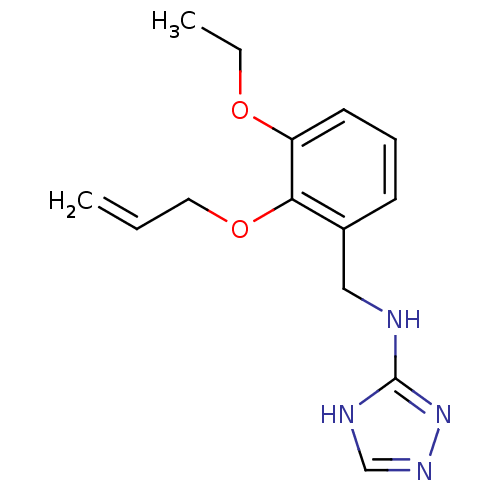
((2-Allyloxy-3-ethoxy-benzyl)-(1H-[1,2,4]triazol-3-...)Show InChI InChI=1S/C14H18N4O2/c1-3-8-20-13-11(6-5-7-12(13)19-4-2)9-15-14-16-10-17-18-14/h3,5-7,10H,1,4,8-9H2,2H3,(H2,15,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| PCBioAssay
| n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics
Curated by PubChem BioAssay
| |
PubChem Bioassay (2012)
BindingDB Entry DOI: 10.7270/Q2HM5725 |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50188966
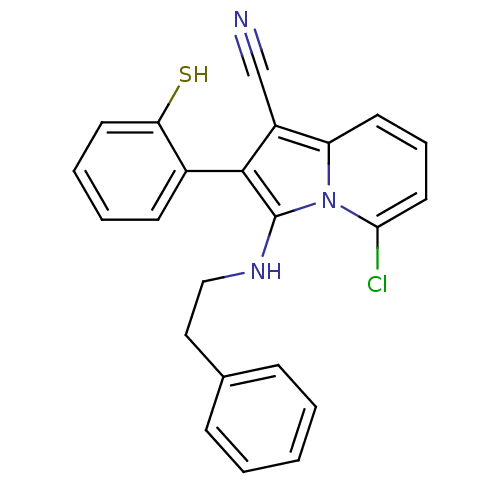
(5-chloro-2-(2-mercaptophenyl)-3-(phenethylamino)in...)Show SMILES Sc1ccccc1-c1c(NCCc2ccccc2)n2c(Cl)cccc2c1C#N |(-2.68,-18.55,;-1.91,-19.88,;-.37,-19.87,;.41,-21.21,;-.37,-22.54,;-1.9,-22.54,;-2.66,-21.2,;-4.2,-21.21,;-5.11,-22.45,;-4.63,-23.92,;-3.13,-24.24,;-2.65,-25.7,;-1.14,-26.02,;-.67,-27.49,;.83,-27.81,;1.86,-26.66,;1.38,-25.19,;-.12,-24.88,;-6.57,-21.98,;-7.9,-22.74,;-7.9,-24.28,;-9.23,-21.97,;-9.23,-20.43,;-7.9,-19.66,;-6.57,-20.43,;-5.11,-19.96,;-4.63,-18.49,;-4.15,-17.03,)| Show InChI InChI=1S/C23H18ClN3S/c24-21-12-6-10-19-18(15-25)22(17-9-4-5-11-20(17)28)23(27(19)21)26-14-13-16-7-2-1-3-8-16/h1-12,26,28H,13-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie
Curated by ChEMBL
| Assay Description
Inhibition of VEGF165-NRP1 interaction by ELISA |
Bioorg Med Chem Lett 16: 3998-4001 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.014
BindingDB Entry DOI: 10.7270/Q20Z72WD |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50189001
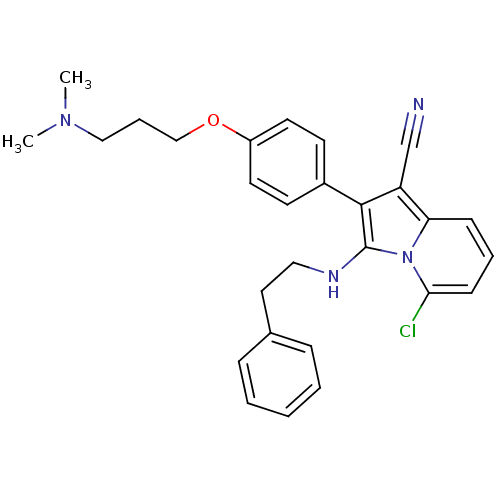
(5-chloro-2-(4-(3-(dimethylamino)propoxy)phenyl)-3-...)Show SMILES CN(C)CCCOc1ccc(cc1)-c1c(NCCc2ccccc2)n2c(Cl)cccc2c1C#N Show InChI InChI=1S/C28H29ClN4O/c1-32(2)18-7-19-34-23-14-12-22(13-15-23)27-24(20-30)25-10-6-11-26(29)33(25)28(27)31-17-16-21-8-4-3-5-9-21/h3-6,8-15,31H,7,16-19H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie
Curated by ChEMBL
| Assay Description
Inhibition of VEGF165-NRP1 interaction by ELISA |
Bioorg Med Chem Lett 16: 3998-4001 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.014
BindingDB Entry DOI: 10.7270/Q20Z72WD |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50313491

((S)-2-(3-(benzo[c][1,2,5]thiadiazole-4-sulfonamido...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-c1sccc1-[#7]S(=O)(=O)c1cccc2nsnc12)-[#6](-[#8])=O |r| Show InChI InChI=1S/C17H19N7O5S3/c18-17(19)20-7-2-4-11(16(26)27)21-15(25)14-10(6-8-30-14)24-32(28,29)12-5-1-3-9-13(12)23-31-22-9/h1,3,5-6,8,11,24H,2,4,7H2,(H,21,25)(H,26,27)(H4,18,19,20)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human biotinylated VEGF-165A expressed in HEK293 cells binding to immobilized recombinant full length human FC-tagged NRP1 ... |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2019.115183
BindingDB Entry DOI: 10.7270/Q2GB27M4 |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50313493
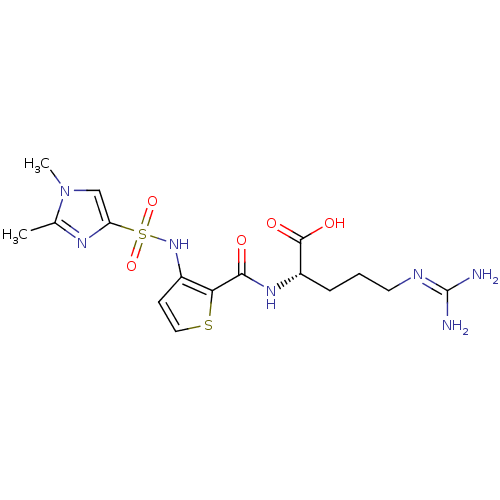
((S)-2-{[3-(1,2-Dimethyl-1H-imidazole-4-sulfonylami...)Show SMILES [#6]-c1nc(cn1-[#6])S(=O)(=O)[#7]-c1ccsc1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#8])=O |r| Show InChI InChI=1S/C16H23N7O5S2/c1-9-20-12(8-23(9)2)30(27,28)22-10-5-7-29-13(10)14(24)21-11(15(25)26)4-3-6-19-16(17)18/h5,7-8,11,22H,3-4,6H2,1-2H3,(H,21,24)(H,25,26)(H4,17,18,19)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]VEGF-A from human NRP1 expressed in pig aortic endothelial cells |
J Med Chem 53: 2215-26 (2010)
Article DOI: 10.1021/jm901755g
BindingDB Entry DOI: 10.7270/Q2154H62 |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50188991
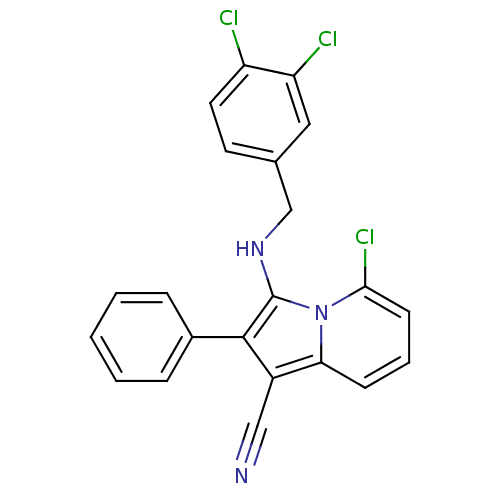
(3-(3,4-dichlorobenzylamino)-5-chloro-2-phenylindol...)Show SMILES Clc1ccc(CNc2c(c(C#N)c3cccc(Cl)n23)-c2ccccc2)cc1Cl Show InChI InChI=1S/C22H14Cl3N3/c23-17-10-9-14(11-18(17)24)13-27-22-21(15-5-2-1-3-6-15)16(12-26)19-7-4-8-20(25)28(19)22/h1-11,27H,13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie
Curated by ChEMBL
| Assay Description
Inhibition of VEGF165-NRP1 interaction by ELISA |
Bioorg Med Chem Lett 16: 3998-4001 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.014
BindingDB Entry DOI: 10.7270/Q20Z72WD |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50313484
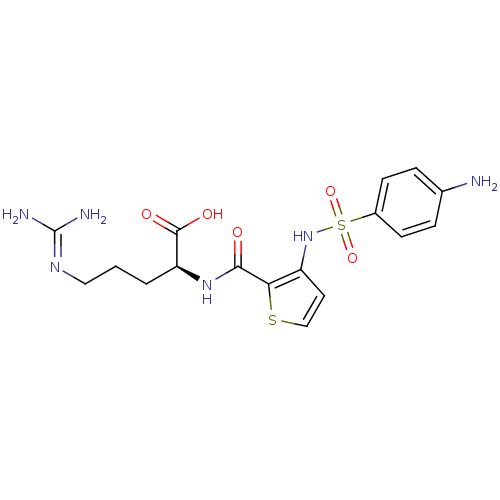
((S)-2-{[3-(4-Amino-benzenesulfonylamino)-thiophene...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-c1sccc1-[#7]S(=O)(=O)c1ccc(-[#7])cc1)-[#6](-[#8])=O |r| Show InChI InChI=1S/C17H22N6O5S2/c18-10-3-5-11(6-4-10)30(27,28)23-12-7-9-29-14(12)15(24)22-13(16(25)26)2-1-8-21-17(19)20/h3-7,9,13,23H,1-2,8,18H2,(H,22,24)(H,25,26)(H4,19,20,21)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]VEGF-A from human NRP1 expressed in pig aortic endothelial cells |
J Med Chem 53: 2215-26 (2010)
Article DOI: 10.1021/jm901755g
BindingDB Entry DOI: 10.7270/Q2154H62 |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50188965
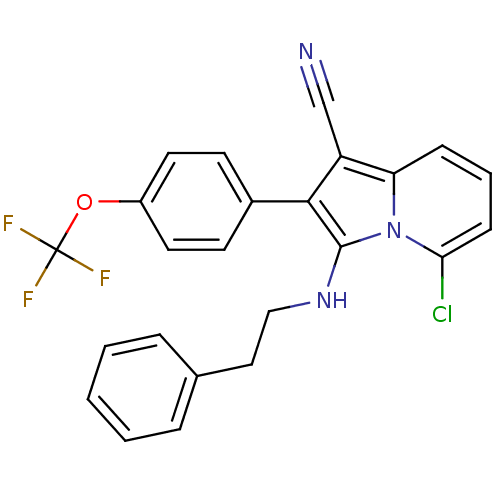
(5-chloro-3-(phenethylamino)-2-(4-(trifluoromethoxy...)Show SMILES FC(F)(F)Oc1ccc(cc1)-c1c(NCCc2ccccc2)n2c(Cl)cccc2c1C#N Show InChI InChI=1S/C24H17ClF3N3O/c25-21-8-4-7-20-19(15-29)22(17-9-11-18(12-10-17)32-24(26,27)28)23(31(20)21)30-14-13-16-5-2-1-3-6-16/h1-12,30H,13-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie
Curated by ChEMBL
| Assay Description
Inhibition of VEGF165-NRP1 interaction by ELISA |
Bioorg Med Chem Lett 16: 3998-4001 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.014
BindingDB Entry DOI: 10.7270/Q20Z72WD |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50188993
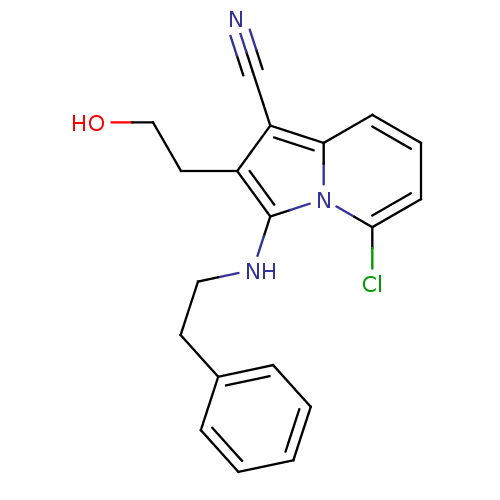
(5-chloro-2-(2-hydroxyethyl)-3-(phenethylamino)indo...)Show InChI InChI=1S/C19H18ClN3O/c20-18-8-4-7-17-16(13-21)15(10-12-24)19(23(17)18)22-11-9-14-5-2-1-3-6-14/h1-8,22,24H,9-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pierre Fabre Urologie
Curated by ChEMBL
| Assay Description
Inhibition of VEGF165-NRP1 interaction by ELISA |
Bioorg Med Chem Lett 16: 3998-4001 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.014
BindingDB Entry DOI: 10.7270/Q20Z72WD |
More data for this
Ligand-Target Pair | |
Neuropilin-1
(Homo sapiens (Human)) | BDBM50313483

(CHEMBL1091089 | H-KPPR-OH)Show SMILES [#7]-[#6]-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](-[#8])=O |r| Show InChI InChI=1S/C22H40N8O5/c23-10-2-1-6-14(24)19(32)30-13-5-9-17(30)20(33)29-12-4-8-16(29)18(31)28-15(21(34)35)7-3-11-27-22(25)26/h14-17H,1-13,23-24H2,(H,28,31)(H,34,35)(H4,25,26,27)/t14-,15-,16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Domainex Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]VEGF-A from human NRP1 expressed in pig aortic endothelial cells |
J Med Chem 53: 2215-26 (2010)
Article DOI: 10.1021/jm901755g
BindingDB Entry DOI: 10.7270/Q2154H62 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data