

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Class D beta-lactamase (Brachyspira pilosicoli) | BDBM50053173 ((2S,3S,5R)-3-Methyl-4,4,7-trioxo-3-[1,2,3]triazol-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Curated by ChEMBL | Assay Description Inhibition of Brachyspira pilosicoli beta-lactamase OXA-63 expressed in Escherichia coli BL21 (DE3) assessed as reduction in nitrocefin hydrolysis by... | Antimicrob Agents Chemother 52: 1264-8 (2008) Article DOI: 10.1128/AAC.00684-07 BindingDB Entry DOI: 10.7270/Q26W9B91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Class D beta-lactamase (Brachyspira pilosicoli) | BDBM50021959 (CHEMBL777 | MM 14151 | US9120808, Clavulanic acid ...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Curated by ChEMBL | Assay Description Inhibition of Brachyspira pilosicoli beta-lactamase OXA-63 expressed in Escherichia coli BL21 (DE3) assessed as reduction in nitrocefin hydrolysis by... | Antimicrob Agents Chemother 52: 1264-8 (2008) Article DOI: 10.1128/AAC.00684-07 BindingDB Entry DOI: 10.7270/Q26W9B91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Class D beta-lactamase (Brachyspira pilosicoli) | BDBM50021954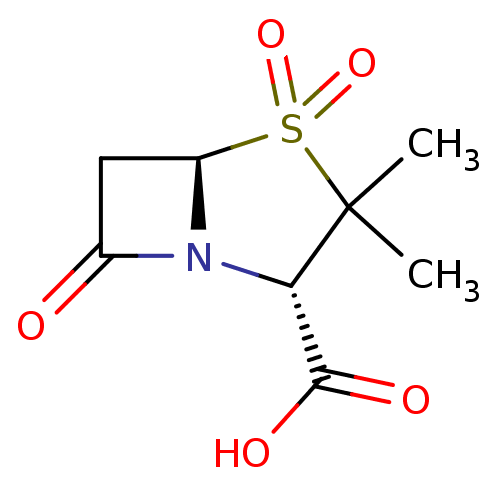 ((2S,5R)-3,3-Dimethyl-4,4,7-trioxo-4lambda*6*-thia-...) | KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Pasteur Curated by ChEMBL | Assay Description Inhibition of Brachyspira pilosicoli beta-lactamase OXA-63 expressed in Escherichia coli BL21 (DE3) assessed as reduction in nitrocefin hydrolysis by... | Antimicrob Agents Chemother 52: 1264-8 (2008) Article DOI: 10.1128/AAC.00684-07 BindingDB Entry DOI: 10.7270/Q26W9B91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| OXA23 carbapenemase (Acinetobacter baumannii) | BDBM50541448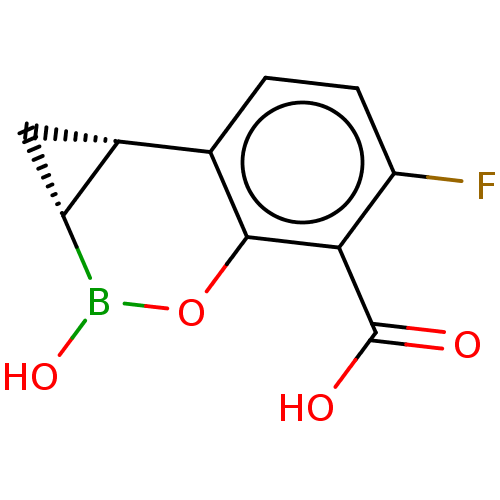 (CHEMBL4633785) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qpex Biopharma, Inc. Curated by ChEMBL | Assay Description Inhibition of Acinetobacter baumannii OXA-23 using nitrocefin as substrate preincubated for 10 mins followed by substrate addition measured every 10 ... | J Med Chem 63: 7491-7507 (2020) Article DOI: 10.1021/acs.jmedchem.9b01976 BindingDB Entry DOI: 10.7270/Q2377D7Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| OXA23 carbapenemase (Acinetobacter baumannii) | BDBM50339145 (CHEMBL1689063 | trans-7-oxo-6-(sulfooxy)-1,6-diaza...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qpex Biopharma, Inc. Curated by ChEMBL | Assay Description Inhibition of Acinetobacter baumannii OXA-23 using nitrocefin as substrate preincubated for 10 mins followed by substrate addition measured every 10 ... | J Med Chem 63: 7491-7507 (2020) Article DOI: 10.1021/acs.jmedchem.9b01976 BindingDB Entry DOI: 10.7270/Q2377D7Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| OXA23 carbapenemase (Acinetobacter baumannii) | BDBM50089084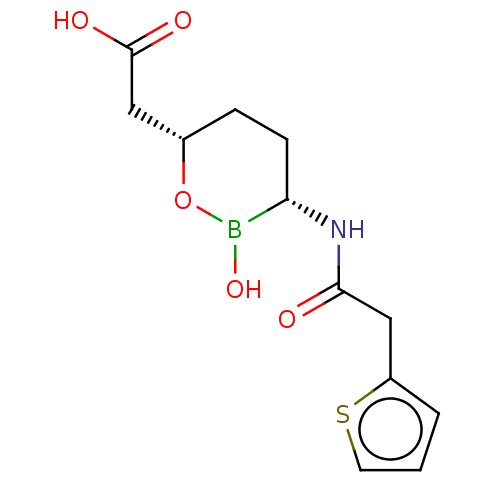 (CHEMBL3317857 | Vaborbactam) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | Article PubMed | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Qpex Biopharma, Inc. Curated by ChEMBL | Assay Description Inhibition of Acinetobacter baumannii OXA-23 using nitrocefin as substrate preincubated for 10 mins followed by substrate addition measured every 10 ... | J Med Chem 63: 7491-7507 (2020) Article DOI: 10.1021/acs.jmedchem.9b01976 BindingDB Entry DOI: 10.7270/Q2377D7Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||