 Found 159 hits Enz. Inhib. hit(s) with Target = 'MAP/microtubule affinity-regulating kinase 2'
Found 159 hits Enz. Inhib. hit(s) with Target = 'MAP/microtubule affinity-regulating kinase 2' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.133 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human MARK2 using KKKVSRSGLYRSPSMPENLNRPR as substrate by [gamma-33P]-ATP assay |
Eur J Med Chem 161: 456-467 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.052
BindingDB Entry DOI: 10.7270/Q2W380MT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.148 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MARK2 using KKKVSRSGLYRSPSMPENLNRPR as substrate by [gamma-33P]-ATP assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2018.02.022
BindingDB Entry DOI: 10.7270/Q2DJ5KB8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM247036
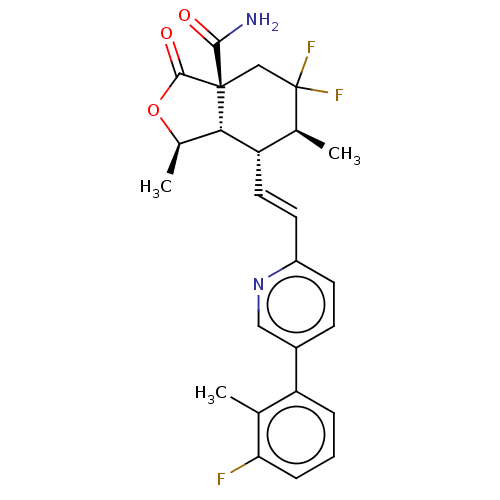
((1R,3aR, 6S,7R,7aS)- 5,5-difluoro- 7-((E)-2-(5- (3...)Show SMILES C[C@H]1OC(=O)[C@@]2(CC(F)(F)[C@@H](C)[C@H](\C=C\c3ccc(cn3)-c3cccc(F)c3C)[C@H]12)C(N)=O |r| Show InChI InChI=1S/C25H25F3N2O3/c1-13-18(5-4-6-20(13)26)16-7-8-17(30-11-16)9-10-19-14(2)25(27,28)12-24(22(29)31)21(19)15(3)33-23(24)32/h4-11,14-15,19,21H,12H2,1-3H3,(H2,29,31)/b10-9+/t14-,15+,19-,21-,24+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.37 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
This assay measures the potency of the inventive compounds as PAR-1 receptor antagonists.Frozen HEK 293 Cells were plated in 384-well PDL coated plat... |
US Patent US9701669 (2017)
BindingDB Entry DOI: 10.7270/Q2PC34DS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM247038
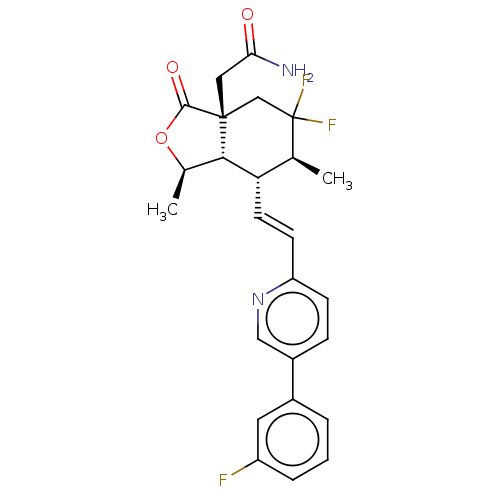
(2-((1R,3aR, 6S,7R,7aS)-5,5- difluoro-7- ((E)-2-(5-...)Show SMILES C[C@H]1OC(=O)[C@]2(CC(N)=O)CC(F)(F)[C@@H](C)[C@H](\C=C\c3ccc(cn3)-c3cccc(F)c3)[C@H]12 |r| Show InChI InChI=1S/C25H25F3N2O3/c1-14-20(9-8-19-7-6-17(12-30-19)16-4-3-5-18(26)10-16)22-15(2)33-23(32)24(22,11-21(29)31)13-25(14,27)28/h3-10,12,14-15,20,22H,11,13H2,1-2H3,(H2,29,31)/b9-8+/t14-,15+,20-,22-,24+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.62 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
This assay measures the potency of the inventive compounds as PAR-1 receptor antagonists.Frozen HEK 293 Cells were plated in 384-well PDL coated plat... |
US Patent US9701669 (2017)
BindingDB Entry DOI: 10.7270/Q2PC34DS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM247035
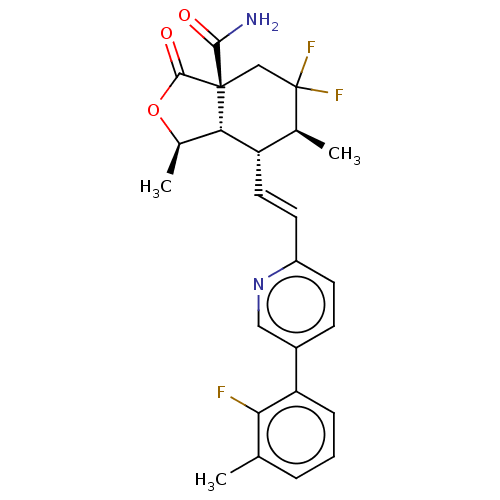
((1R,3aR,6S,7R, 7aS)-5,5-difluoro- 7-((E)-2-(5- (2-...)Show SMILES C[C@H]1OC(=O)[C@@]2(CC(F)(F)[C@@H](C)[C@H](\C=C\c3ccc(cn3)-c3cccc(C)c3F)[C@H]12)C(N)=O |r| Show InChI InChI=1S/C25H25F3N2O3/c1-13-5-4-6-19(21(13)26)16-7-8-17(30-11-16)9-10-18-14(2)25(27,28)12-24(22(29)31)20(18)15(3)33-23(24)32/h4-11,14-15,18,20H,12H2,1-3H3,(H2,29,31)/b10-9+/t14-,15+,18-,20-,24+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
This assay measures the potency of the inventive compounds as PAR-1 receptor antagonists.Frozen HEK 293 Cells were plated in 384-well PDL coated plat... |
US Patent US9701669 (2017)
BindingDB Entry DOI: 10.7270/Q2PC34DS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM247039
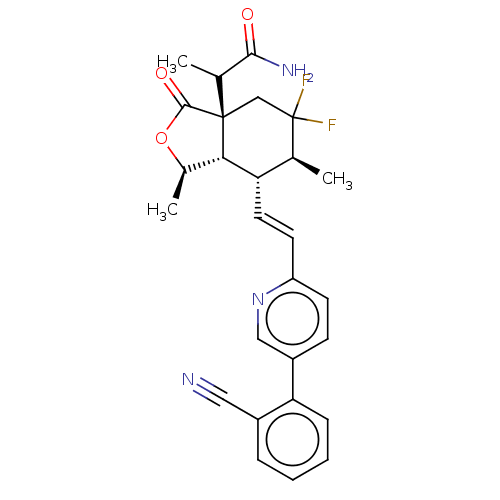
(2-((1R,3aR, 6S,7R,7aS)-7- ((E)-2-(5-(2- cyanopheny...)Show SMILES CC(C(N)=O)[C@@]12CC(F)(F)[C@@H](C)[C@H](\C=C\c3ccc(cn3)-c3ccccc3C#N)[C@@H]1[C@@H](C)OC2=O |r| Show InChI InChI=1S/C27H27F2N3O3/c1-15-21(11-10-20-9-8-19(13-32-20)22-7-5-4-6-18(22)12-30)23-17(3)35-25(34)26(23,14-27(15,28)29)16(2)24(31)33/h4-11,13,15-17,21,23H,14H2,1-3H3,(H2,31,33)/b11-10+/t15-,16?,17+,21-,23-,26-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.07 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
This assay measures the potency of the inventive compounds as PAR-1 receptor antagonists.Frozen HEK 293 Cells were plated in 384-well PDL coated plat... |
US Patent US9701669 (2017)
BindingDB Entry DOI: 10.7270/Q2PC34DS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM247047
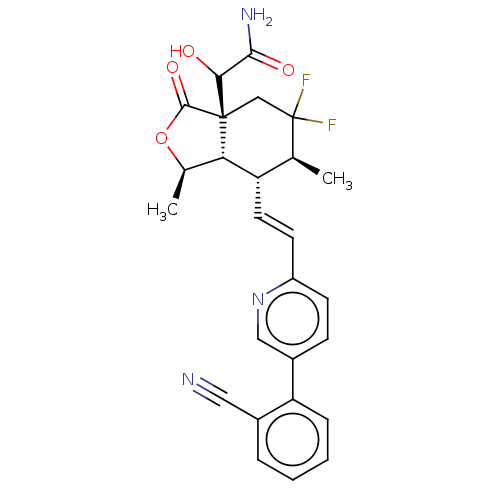
((S and R)-2- ((1R,3aR,6S, 7R,7aS)-7- ((E)-2-(5-(2-...)Show SMILES C[C@H]1OC(=O)[C@@]2(CC(F)(F)[C@@H](C)[C@H](\C=C\c3ccc(cn3)-c3ccccc3C#N)[C@H]12)C(O)C(N)=O |r| Show InChI InChI=1S/C26H25F2N3O4/c1-14-19(10-9-18-8-7-17(12-31-18)20-6-4-3-5-16(20)11-29)21-15(2)35-24(34)25(21,13-26(14,27)28)22(32)23(30)33/h3-10,12,14-15,19,21-22,32H,13H2,1-2H3,(H2,30,33)/b10-9+/t14-,15+,19-,21-,22?,25+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.41 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
This assay measures the potency of the inventive compounds as PAR-1 receptor antagonists.Frozen HEK 293 Cells were plated in 384-well PDL coated plat... |
US Patent US9701669 (2017)
BindingDB Entry DOI: 10.7270/Q2PC34DS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM247039

(2-((1R,3aR, 6S,7R,7aS)-7- ((E)-2-(5-(2- cyanopheny...)Show SMILES CC(C(N)=O)[C@@]12CC(F)(F)[C@@H](C)[C@H](\C=C\c3ccc(cn3)-c3ccccc3C#N)[C@@H]1[C@@H](C)OC2=O |r| Show InChI InChI=1S/C27H27F2N3O3/c1-15-21(11-10-20-9-8-19(13-32-20)22-7-5-4-6-18(22)12-30)23-17(3)35-25(34)26(23,14-27(15,28)29)16(2)24(31)33/h4-11,13,15-17,21,23H,14H2,1-3H3,(H2,31,33)/b11-10+/t15-,16?,17+,21-,23-,26-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.43 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
This assay measures the potency of the inventive compounds as PAR-1 receptor antagonists.Frozen HEK 293 Cells were plated in 384-well PDL coated plat... |
US Patent US9701669 (2017)
BindingDB Entry DOI: 10.7270/Q2PC34DS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM247044
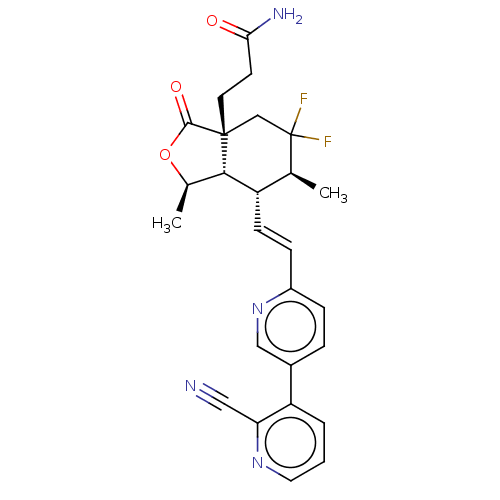
(3-((1R,3aR,6S, 7R,7aS)-7 -((E)- 2- (2'-cyano-[...)Show SMILES C[C@H]1OC(=O)[C@]2(CCC(N)=O)CC(F)(F)[C@@H](C)[C@H](\C=C\c3ccc(cn3)-c3cccnc3C#N)[C@H]12 |r| Show InChI InChI=1S/C26H26F2N4O3/c1-15-19(8-7-18-6-5-17(13-32-18)20-4-3-11-31-21(20)12-29)23-16(2)35-24(34)25(23,10-9-22(30)33)14-26(15,27)28/h3-8,11,13,15-16,19,23H,9-10,14H2,1-2H3,(H2,30,33)/b8-7+/t15-,16+,19-,23-,25+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6.34 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
This assay measures the potency of the inventive compounds as PAR-1 receptor antagonists.Frozen HEK 293 Cells were plated in 384-well PDL coated plat... |
US Patent US9701669 (2017)
BindingDB Entry DOI: 10.7270/Q2PC34DS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM247037
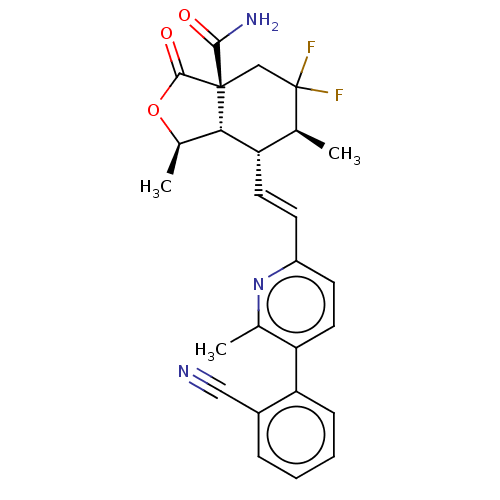
((1R,3aR,6S,7R, 7aS)-7-((E)-2-(5- (2-cyanophenyl)- ...)Show SMILES C[C@H]1OC(=O)[C@@]2(CC(F)(F)[C@@H](C)[C@H](\C=C\c3ccc(c(C)n3)-c3ccccc3C#N)[C@H]12)C(N)=O |r| Show InChI InChI=1S/C26H25F2N3O3/c1-14-19(22-16(3)34-24(33)25(22,23(30)32)13-26(14,27)28)10-8-18-9-11-20(15(2)31-18)21-7-5-4-6-17(21)12-29/h4-11,14,16,19,22H,13H2,1-3H3,(H2,30,32)/b10-8+/t14-,16+,19-,22-,25+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6.92 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
This assay measures the potency of the inventive compounds as PAR-1 receptor antagonists.Frozen HEK 293 Cells were plated in 384-well PDL coated plat... |
US Patent US9701669 (2017)
BindingDB Entry DOI: 10.7270/Q2PC34DS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM247042
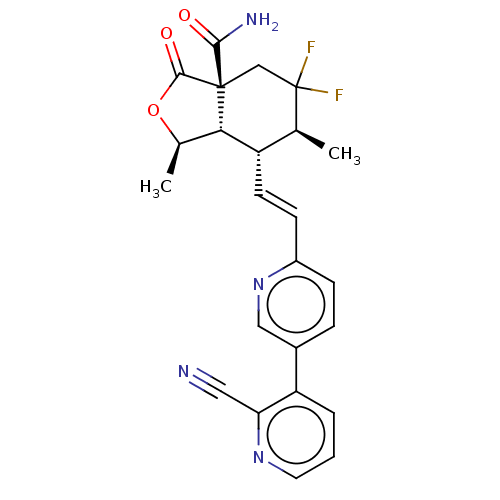
(1R,3aR,6S, 7R,7aS)-7-((E)-2- (2'-cyano-[3,3�...)Show SMILES C[C@H]1OC(=O)[C@@]2(CC(F)(F)[C@@H](C)[C@H](\C=C\c3ccc(cn3)-c3cccnc3C#N)[C@H]12)C(N)=O |r| Show InChI InChI=1S/C24H22F2N4O3/c1-13-17(20-14(2)33-22(32)23(20,21(28)31)12-24(13,25)26)8-7-16-6-5-15(11-30-16)18-4-3-9-29-19(18)10-27/h3-9,11,13-14,17,20H,12H2,1-2H3,(H2,28,31)/b8-7+/t13-,14+,17-,20-,23+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 9.68 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
This assay measures the potency of the inventive compounds as PAR-1 receptor antagonists.Frozen HEK 293 Cells were plated in 384-well PDL coated plat... |
US Patent US9701669 (2017)
BindingDB Entry DOI: 10.7270/Q2PC34DS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM247039

(2-((1R,3aR, 6S,7R,7aS)-7- ((E)-2-(5-(2- cyanopheny...)Show SMILES CC(C(N)=O)[C@@]12CC(F)(F)[C@@H](C)[C@H](\C=C\c3ccc(cn3)-c3ccccc3C#N)[C@@H]1[C@@H](C)OC2=O |r| Show InChI InChI=1S/C27H27F2N3O3/c1-15-21(11-10-20-9-8-19(13-32-20)22-7-5-4-6-18(22)12-30)23-17(3)35-25(34)26(23,14-27(15,28)29)16(2)24(31)33/h4-11,13,15-17,21,23H,14H2,1-3H3,(H2,31,33)/b11-10+/t15-,16?,17+,21-,23-,26-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 11.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
This assay measures the potency of the inventive compounds as PAR-1 receptor antagonists.Frozen HEK 293 Cells were plated in 384-well PDL coated plat... |
US Patent US9701669 (2017)
BindingDB Entry DOI: 10.7270/Q2PC34DS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM247043

(1-((1R,3aS,6S, 7R,7aS)-5,5- difluoro-7- ((E)-2-(5-...)Show SMILES C[C@H]1OC(=O)[C@@]2(CC(F)(F)[C@@H](C)[C@H](\C=C\c3ccc(cn3)-c3cccc(F)c3)[C@H]12)N1CC(C1)C(N)=O |r| Show InChI InChI=1S/C27H28F3N3O3/c1-15-22(9-8-21-7-6-18(11-32-21)17-4-3-5-20(28)10-17)23-16(2)36-25(35)26(23,14-27(15,29)30)33-12-19(13-33)24(31)34/h3-11,15-16,19,22-23H,12-14H2,1-2H3,(H2,31,34)/b9-8+/t15-,16+,22-,23-,26-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 13.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
This assay measures the potency of the inventive compounds as PAR-1 receptor antagonists.Frozen HEK 293 Cells were plated in 384-well PDL coated plat... |
US Patent US9701669 (2017)
BindingDB Entry DOI: 10.7270/Q2PC34DS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM247046

(2-((1R,3aR,6S, 7R,7aS)-7-((E)- 2- (2'-cyano-[3...)Show SMILES C[C@H]1OC(=O)[C@]2(CC(N)=O)CC(F)(F)[C@@H](C)[C@H](\C=C\c3ccc(cn3)-c3cccnc3C#N)[C@H]12 |r| Show InChI InChI=1S/C25H24F2N4O3/c1-14-18(22-15(2)34-23(33)24(22,10-21(29)32)13-25(14,26)27)8-7-17-6-5-16(12-31-17)19-4-3-9-30-20(19)11-28/h3-9,12,14-15,18,22H,10,13H2,1-2H3,(H2,29,32)/b8-7+/t14-,15+,18-,22-,24+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 21.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
This assay measures the potency of the inventive compounds as PAR-1 receptor antagonists.Frozen HEK 293 Cells were plated in 384-well PDL coated plat... |
US Patent US9701669 (2017)
BindingDB Entry DOI: 10.7270/Q2PC34DS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM247045
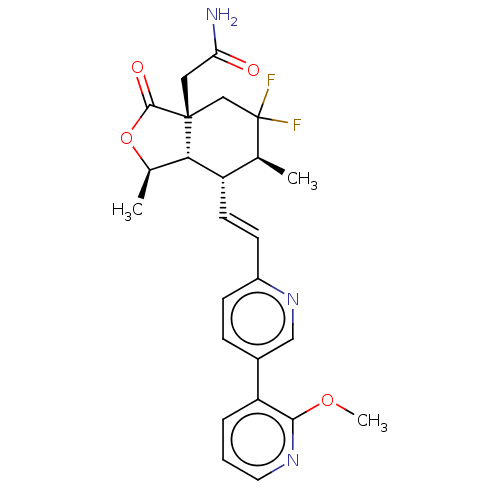
(2-((1R,3aR,6S, 7R,7aS)-5,5- difluoro-7- ((E)-2-(2&...)Show SMILES COc1ncccc1-c1ccc(\C=C\[C@@H]2[C@@H]3[C@@H](C)OC(=O)[C@]3(CC(N)=O)CC(F)(F)[C@H]2C)nc1 |r| Show InChI InChI=1S/C25H27F2N3O4/c1-14-18(21-15(2)34-23(32)24(21,11-20(28)31)13-25(14,26)27)9-8-17-7-6-16(12-30-17)19-5-4-10-29-22(19)33-3/h4-10,12,14-15,18,21H,11,13H2,1-3H3,(H2,28,31)/b9-8+/t14-,15+,18-,21-,24+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
This assay measures the potency of the inventive compounds as PAR-1 receptor antagonists.Frozen HEK 293 Cells were plated in 384-well PDL coated plat... |
US Patent US9701669 (2017)
BindingDB Entry DOI: 10.7270/Q2PC34DS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM50462709
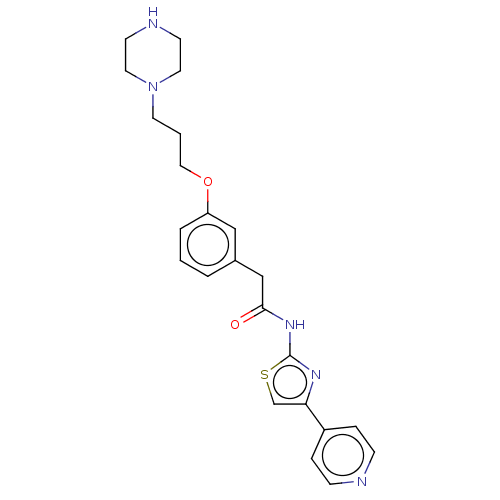
(CHEMBL4245507)Show SMILES O=C(Cc1cccc(OCCCN2CCNCC2)c1)Nc1nc(cs1)-c1ccncc1 Show InChI InChI=1S/C23H27N5O2S/c29-22(27-23-26-21(17-31-23)19-5-7-24-8-6-19)16-18-3-1-4-20(15-18)30-14-2-11-28-12-9-25-10-13-28/h1,3-8,15,17,25H,2,9-14,16H2,(H,26,27,29) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of PAR-1B (unknown origin) |
Bioorg Med Chem Lett 28: 2616-2621 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.043
BindingDB Entry DOI: 10.7270/Q2XP77MH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM50204693
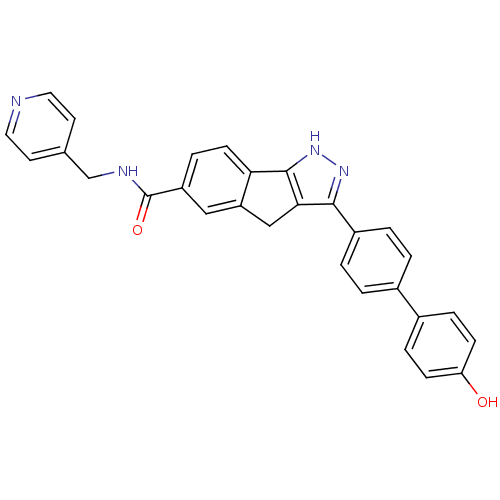
(3-(4'-hydroxy-biphenyl-4-yl)-1,4-dihydro-indeno[1,...)Show SMILES Oc1ccc(cc1)-c1ccc(cc1)-c1n[nH]c-2c1Cc1cc(ccc-21)C(=O)NCc1ccncc1 Show InChI InChI=1S/C29H22N4O2/c34-24-8-5-20(6-9-24)19-1-3-21(4-2-19)27-26-16-23-15-22(7-10-25(23)28(26)33-32-27)29(35)31-17-18-11-13-30-14-12-18/h1-15,34H,16-17H2,(H,31,35)(H,32,33) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of EMK by radiometric assay |
Bioorg Med Chem 15: 2759-67 (2007)
Article DOI: 10.1016/j.bmc.2007.01.012
BindingDB Entry DOI: 10.7270/Q2XS5V22 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM50218709
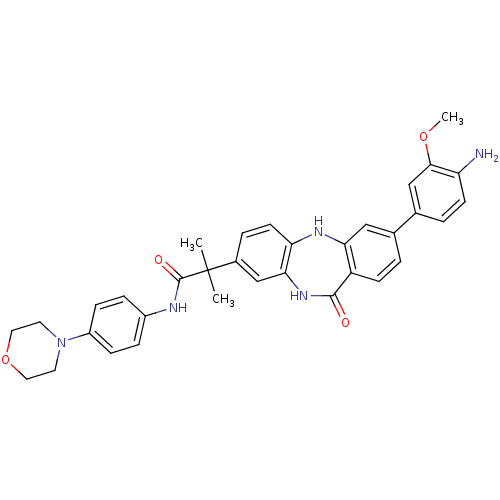
(2-[3-(3-methoxy-4-nitrophenyl)-11-oxo-10,11-dihydr...)Show SMILES COc1cc(ccc1N)-c1ccc2c(Nc3ccc(cc3NC2=O)C(C)(C)C(=O)Nc2ccc(cc2)N2CCOCC2)c1 Show InChI InChI=1S/C34H35N5O4/c1-34(2,33(41)36-24-7-9-25(10-8-24)39-14-16-43-17-15-39)23-6-13-28-30(20-23)38-32(40)26-11-4-21(18-29(26)37-28)22-5-12-27(35)31(19-22)42-3/h4-13,18-20,37H,14-17,35H2,1-3H3,(H,36,41)(H,38,40) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant EMK by radiometric assay |
J Med Chem 50: 4162-76 (2007)
Article DOI: 10.1021/jm070105d
BindingDB Entry DOI: 10.7270/Q22J6BKS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM50401152
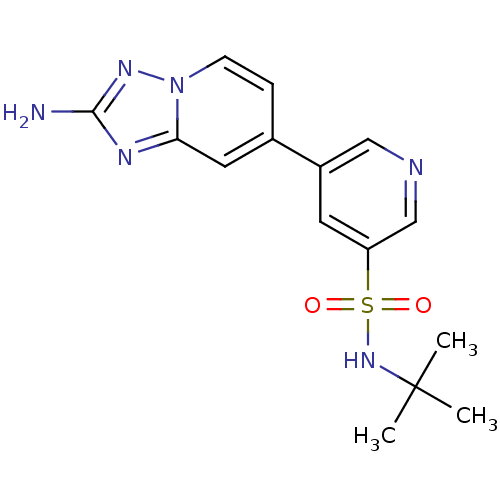
(CHEMBL2205766)Show SMILES CC(C)(C)NS(=O)(=O)c1cncc(c1)-c1ccn2nc(N)nc2c1 Show InChI InChI=1S/C15H18N6O2S/c1-15(2,3)20-24(22,23)12-6-11(8-17-9-12)10-4-5-21-13(7-10)18-14(16)19-21/h4-9,20H,1-3H3,(H2,16,19) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cellzome Ltd
Curated by ChEMBL
| Assay Description
Inhibition of MARK2 |
Bioorg Med Chem Lett 22: 4613-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.090
BindingDB Entry DOI: 10.7270/Q2HQ412B |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM50135286

(CHEMBL3745885)Show SMILES Cn1c2nc(Nc3ccc4[nH]ccc4c3)ncc2cc(c1=O)S(=O)(=O)c1ccc(F)cc1F Show InChI InChI=1S/C22H15F2N5O3S/c1-29-20-13(9-19(21(29)30)33(31,32)18-5-2-14(23)10-16(18)24)11-26-22(28-20)27-15-3-4-17-12(8-15)6-7-25-17/h2-11,25H,1H3,(H,26,27,28) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
| Assay Description
Inhibition of human MARK2 using [KKKVSRSGLYRSPSMPENLNRPR] as substrate |
Bioorg Med Chem 24: 521-44 (2016)
Article DOI: 10.1016/j.bmc.2015.11.045
BindingDB Entry DOI: 10.7270/Q24Q7WT8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM50519662
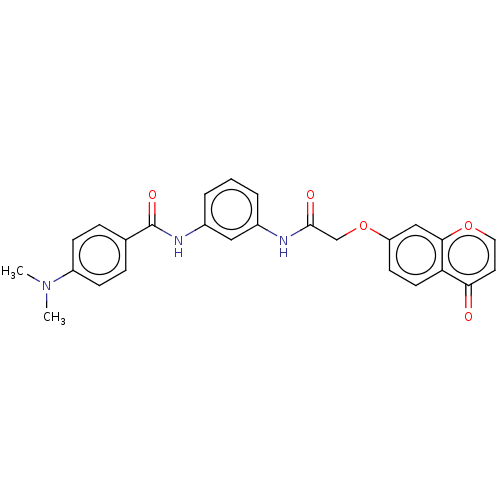
(CHEMBL4438748)Show SMILES CN(C)c1ccc(cc1)C(=O)Nc1cccc(NC(=O)COc2ccc3c(c2)occc3=O)c1 Show InChI InChI=1S/C26H23N3O5/c1-29(2)20-8-6-17(7-9-20)26(32)28-19-5-3-4-18(14-19)27-25(31)16-34-21-10-11-22-23(30)12-13-33-24(22)15-21/h3-15H,16H2,1-2H3,(H,27,31)(H,28,32) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full-length human PAR1Balpha using KKKVSRSGLYRSP as substrate measured after 40 mins in presence of [gamma33P]ATP by radiom... |
J Med Chem 62: 10691-10710 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01143
BindingDB Entry DOI: 10.7270/Q2MC93FG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM50218700
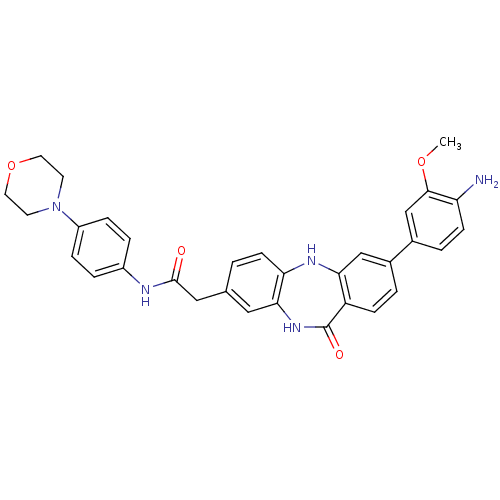
(2-[3-(3-methoxy-4-nitrophenyl)-11-oxo-10,11-dihydr...)Show SMILES COc1cc(ccc1N)-c1ccc2c(Nc3ccc(CC(=O)Nc4ccc(cc4)N4CCOCC4)cc3NC2=O)c1 Show InChI InChI=1S/C32H31N5O4/c1-40-30-19-22(4-10-26(30)33)21-3-9-25-28(18-21)35-27-11-2-20(16-29(27)36-32(25)39)17-31(38)34-23-5-7-24(8-6-23)37-12-14-41-15-13-37/h2-11,16,18-19,35H,12-15,17,33H2,1H3,(H,34,38)(H,36,39) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant EMK by radiometric assay |
J Med Chem 50: 4162-76 (2007)
Article DOI: 10.1021/jm070105d
BindingDB Entry DOI: 10.7270/Q22J6BKS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM50359359

(CHEMBL1929238)Show SMILES CN(C)CCN1CCN(CCC1=O)C(=O)c1cc(sc1NC(=O)Nc1cccc(Cl)c1Cl)C(C)(C)C Show InChI InChI=1S/C25H33Cl2N5O3S/c1-25(2,3)19-15-16(22(36-19)29-24(35)28-18-8-6-7-17(26)21(18)27)23(34)32-10-9-20(33)31(13-14-32)12-11-30(4)5/h6-8,15H,9-14H2,1-5H3,(H2,28,29,35) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of MARK2/PAR-1Balpha |
Bioorg Med Chem Lett 21: 7155-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.078
BindingDB Entry DOI: 10.7270/Q2NC61NH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of MARK2/PAR-1Balpha |
Bioorg Med Chem Lett 21: 7155-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.078
BindingDB Entry DOI: 10.7270/Q2NC61NH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | CHEMBL5278465
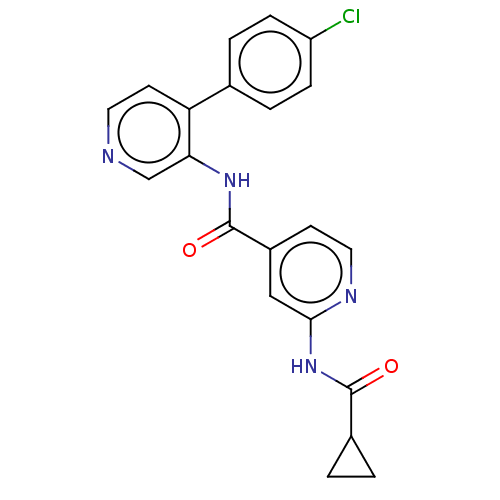
| PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM50204694
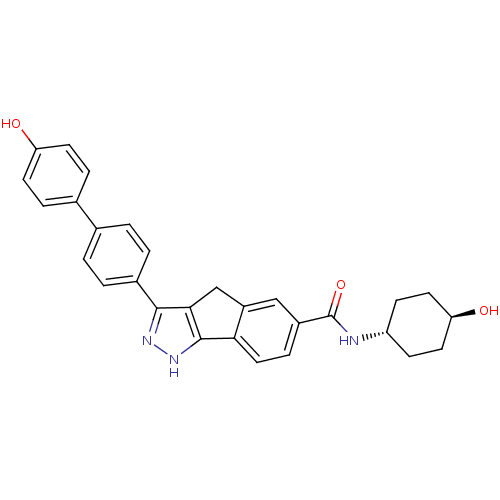
(3-(4'-HYDROXYBIPHENYL-4-YL)-N-(4-HYDROXYCYCLOHEXYL...)Show SMILES O[C@H]1CC[C@@H](CC1)NC(=O)c1ccc-2c(Cc3c(n[nH]c-23)-c2ccc(cc2)-c2ccc(O)cc2)c1 |wU:4.7,wD:1.0,(19.77,-1.9,;21.02,-1.01,;22.42,-1.66,;23.69,-.77,;23.53,.76,;22.14,1.41,;20.89,.52,;24.79,1.65,;26.19,1.01,;26.33,-.53,;27.44,1.9,;27.3,3.44,;28.55,4.33,;29.95,3.7,;30.1,2.15,;31.61,1.81,;32.4,3.14,;33.83,3.77,;33.68,5.31,;32.16,5.65,;31.37,4.31,;35.16,2.99,;35.15,1.44,;36.46,.66,;37.81,1.42,;37.82,2.96,;36.49,3.74,;39.13,.64,;39.12,-.9,;40.44,-1.67,;41.78,-.91,;43.11,-1.69,;41.78,.63,;40.46,1.4,;28.85,1.26,)| Show InChI InChI=1S/C29H27N3O3/c33-23-10-5-18(6-11-23)17-1-3-19(4-2-17)27-26-16-21-15-20(7-14-25(21)28(26)32-31-27)29(35)30-22-8-12-24(34)13-9-22/h1-7,10-11,14-15,22,24,33-34H,8-9,12-13,16H2,(H,30,35)(H,31,32)/t22-,24- | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of EMK by radiometric assay |
Bioorg Med Chem 15: 2759-67 (2007)
Article DOI: 10.1016/j.bmc.2007.01.012
BindingDB Entry DOI: 10.7270/Q2XS5V22 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM50537742
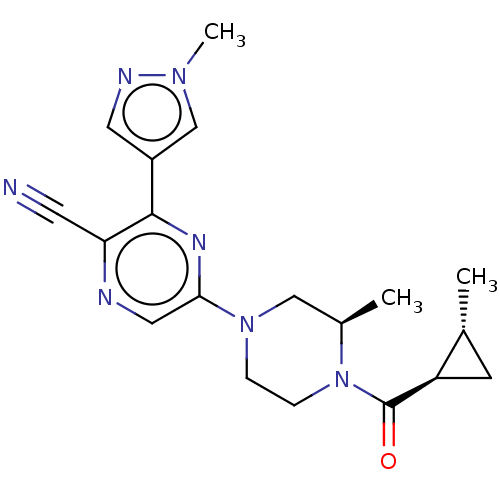
(CHEMBL4634634 | US11179389, Compound 1-14)Show SMILES C[C@@H]1C[C@H]1C(=O)N1CCN(C[C@H]1C)c1cnc(C#N)c(n1)-c1cnn(C)c1 |r| Show InChI InChI=1S/C19H23N7O/c1-12-6-15(12)19(27)26-5-4-25(10-13(26)2)17-9-21-16(7-20)18(23-17)14-8-22-24(3)11-14/h8-9,11-13,15H,4-6,10H2,1-3H3/t12-,13-,15-/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human GST-tagged MARK2 expressed in baculovirus expression system using serine/threonine-21 peptide as substrat... |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126715
BindingDB Entry DOI: 10.7270/Q2HM5CZG |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM50402020
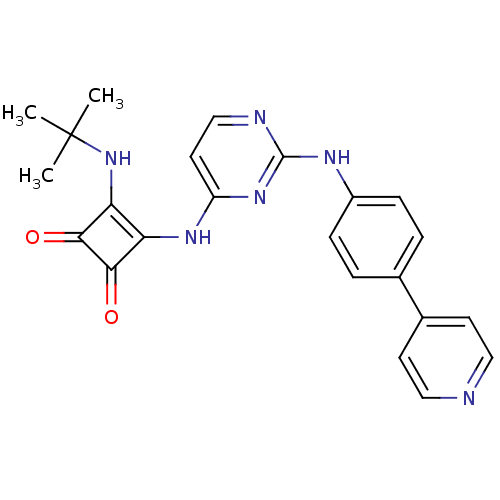
(CHEMBL2205426)Show SMILES CC(C)(C)Nc1c(Nc2ccnc(Nc3ccc(cc3)-c3ccncc3)n2)c(=O)c1=O Show InChI InChI=1S/C23H22N6O2/c1-23(2,3)29-19-18(20(30)21(19)31)27-17-10-13-25-22(28-17)26-16-6-4-14(5-7-16)15-8-11-24-12-9-15/h4-13,29H,1-3H3,(H2,25,26,27,28) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant EMK after 1 hr by scintillation counter analysis in presence of gamma-[33P]ATP |
Bioorg Med Chem Lett 22: 7615-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.009
BindingDB Entry DOI: 10.7270/Q2XK8GQ3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM50224883
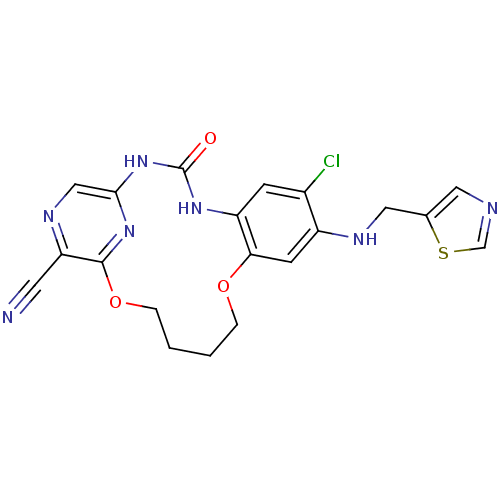
(7-chloro-3-oxo-8-[(thiazol-5-ylmethyl)-amino]-11,1...)Show SMILES Clc1cc2NC(=O)Nc3cnc(C#N)c(OCCCCOc2cc1NCc1cncs1)n3 Show InChI InChI=1S/C20H18ClN7O3S/c21-13-5-15-17(6-14(13)24-9-12-8-23-11-32-12)30-3-1-2-4-31-19-16(7-22)25-10-18(27-19)28-20(29)26-15/h5-6,8,10-11,24H,1-4,9H2,(H2,26,27,28,29) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of EMK |
Bioorg Med Chem Lett 17: 6593-601 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.063
BindingDB Entry DOI: 10.7270/Q2X067WT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM25118
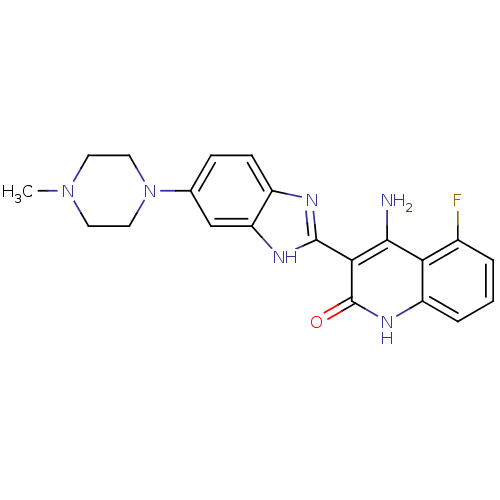
((3Z)-4-amino-5-fluoro-3-[5-(4-methylpiperazino)-1,...)Show SMILES CN1CCN(CC1)c1ccc2nc([nH]c2c1)-c1c(N)c2c(F)cccc2[nH]c1=O Show InChI InChI=1S/C21H21FN6O/c1-27-7-9-28(10-8-27)12-5-6-14-16(11-12)25-20(24-14)18-19(23)17-13(22)3-2-4-15(17)26-21(18)29/h2-6,11H,7-10H2,1H3,(H,24,25)(H3,23,26,29) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2W37TQQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM6866
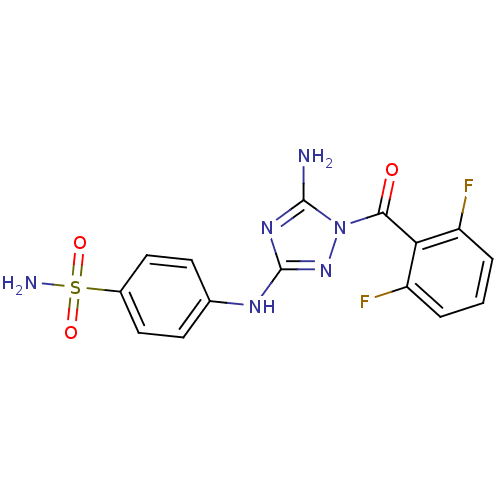
(1-Acyl-1H-[1,2,4]triazole-3,5-diamine Analogue 3b ...)Show SMILES Nc1nc(Nc2ccc(cc2)S(N)(=O)=O)nn1C(=O)c1c(F)cccc1F Show InChI InChI=1S/C15H12F2N6O3S/c16-10-2-1-3-11(17)12(10)13(24)23-14(18)21-15(22-23)20-8-4-6-9(7-5-8)27(19,25)26/h1-7H,(H2,19,25,26)(H3,18,20,21,22) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2W37TQQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM31095
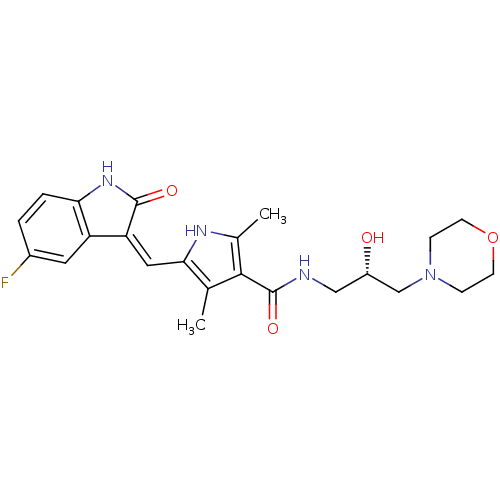
(5-[(Z)-(5-fluoranyl-2-oxidanylidene-1H-indol-3-yli...)Show SMILES Cc1[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c(C)c1C(=O)NC[C@H](O)CN1CCOCC1 Show InChI InChI=1S/C23H27FN4O4/c1-13-20(10-18-17-9-15(24)3-4-19(17)27-22(18)30)26-14(2)21(13)23(31)25-11-16(29)12-28-5-7-32-8-6-28/h3-4,9-10,16,26,29H,5-8,11-12H2,1-2H3,(H,25,31)(H,27,30)/b18-10-/t16-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2W37TQQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM31096

(CHEMBL290084 | Staurosporine | cid_451705)Show SMILES CN[C@H]1C[C@@H]2O[C@](C)([C@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2W37TQQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM4814

(CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2W37TQQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2W37TQQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Average Binding Constant for MARK2; NA=Not Active at 10 uM |
Nat Biotechnol 23: 329-36 (2005)
Article DOI: 10.1038/nbt1068
BindingDB Entry DOI: 10.7270/Q2V69J3T |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM50024294
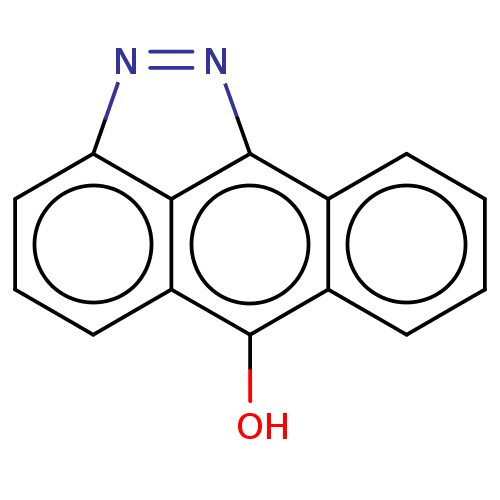
(SP-600125)Show InChI InChI=1S/C14H8N2O/c17-14-9-5-2-1-4-8(9)13-12-10(14)6-3-7-11(12)15-16-13/h1-7,17H | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Average Binding Constant for MARK2; NA=Not Active at 10 uM |
Nat Biotechnol 23: 329-36 (2005)
Article DOI: 10.1038/nbt1068
BindingDB Entry DOI: 10.7270/Q2V69J3T |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM50308060
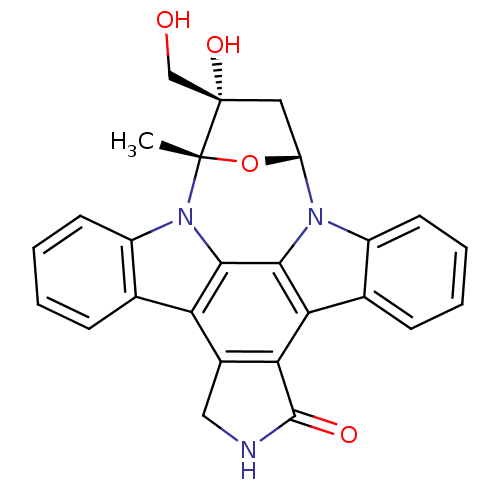
(16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...)Show SMILES C[C@]12O[C@H](C[C@]1(O)CO)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to MARK2 |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM50326053
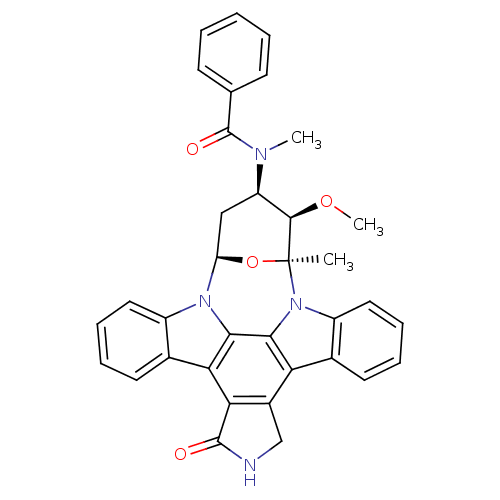
(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to MARK2 |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM50026612

(BIBF-1120 | Nintedanib | US10981896, Compound Nint...)Show SMILES COC(=O)c1ccc2\C(=C(\Nc3ccc(cc3)N(C)C(=O)CN3CCN(C)CC3)c3ccccc3)C(=O)Nc2c1 Show InChI InChI=1S/C31H33N5O4/c1-34-15-17-36(18-16-34)20-27(37)35(2)24-12-10-23(11-13-24)32-29(21-7-5-4-6-8-21)28-25-14-9-22(31(39)40-3)19-26(25)33-30(28)38/h4-14,19,32H,15-18,20H2,1-3H3,(H,33,38)/b29-28- | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for MARK2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM50308060

(16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...)Show SMILES C[C@]12O[C@H](C[C@]1(O)CO)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for MARK2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for MARK2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM50326053

(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for MARK2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM50326053

(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for MARK2 kinase domain |
Nat Biotechnol 26: 127-32 (2008)
Article DOI: 10.1038/nbt1358
BindingDB Entry DOI: 10.7270/Q2TT4RX2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for MARK2 kinase domain |
Nat Biotechnol 26: 127-32 (2008)
Article DOI: 10.1038/nbt1358
BindingDB Entry DOI: 10.7270/Q2TT4RX2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM4814

(CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Average Binding Constant for MARK2; NA=Not Active at 10 uM |
Nat Biotechnol 23: 329-36 (2005)
Article DOI: 10.1038/nbt1068
BindingDB Entry DOI: 10.7270/Q2V69J3T |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM17055

((18S)-18-[(dimethylamino)methyl]-17-oxa-4,14,21-tr...)Show SMILES CN(C)C[C@@H]1CCn2cc(C3=C(C(=O)NC3=O)c3cn(CCO1)c1ccccc31)c1ccccc21 |r,t:10| Show InChI InChI=1S/C28H28N4O3/c1-30(2)15-18-11-12-31-16-21(19-7-3-5-9-23(19)31)25-26(28(34)29-27(25)33)22-17-32(13-14-35-18)24-10-6-4-8-20(22)24/h3-10,16-18H,11-15H2,1-2H3,(H,29,33,34)/t18-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Average Binding Constant for MARK2; NA=Not Active at 10 uM |
Nat Biotechnol 23: 329-36 (2005)
Article DOI: 10.1038/nbt1068
BindingDB Entry DOI: 10.7270/Q2V69J3T |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to MARK2 |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM50300690

(1-(5-Tert-Butyl-1,2-Oxazol-3-Yl)-3-(4-{7-[2-(Morph...)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(cc2)-c2cn3c(n2)sc2cc(OCCN4CCOCC4)ccc32)no1 Show InChI InChI=1S/C29H32N6O4S/c1-29(2,3)25-17-26(33-39-25)32-27(36)30-20-6-4-19(5-7-20)22-18-35-23-9-8-21(16-24(23)40-28(35)31-22)38-15-12-34-10-13-37-14-11-34/h4-9,16-18H,10-15H2,1-3H3,(H2,30,32,33,36) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to MARK2 |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase MARK2
(Homo sapiens (Human)) | BDBM13535
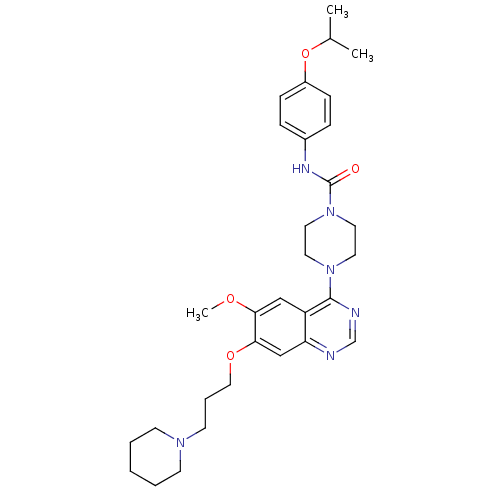
(4-[6-methoxy-7-(3-piperidin-1-ylpropoxy)quinazolin...)Show SMILES COc1cc2c(ncnc2cc1OCCCN1CCCCC1)N1CCN(CC1)C(=O)Nc1ccc(OC(C)C)cc1 Show InChI InChI=1S/C31H42N6O4/c1-23(2)41-25-10-8-24(9-11-25)34-31(38)37-17-15-36(16-18-37)30-26-20-28(39-3)29(21-27(26)32-22-33-30)40-19-7-14-35-12-5-4-6-13-35/h8-11,20-23H,4-7,12-19H2,1-3H3,(H,34,38) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to MARK2 |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data