

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50088301 ((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles Curated by ChEMBL | Assay Description Displacement of [125I]MCP1 from CCR2/CXCR4 (unknown origin) expressed in CHOK1 cells | J Biol Chem 282: 30062-9 (2007) Article DOI: 10.1074/jbc.M705302200 BindingDB Entry DOI: 10.7270/Q27M07Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50035696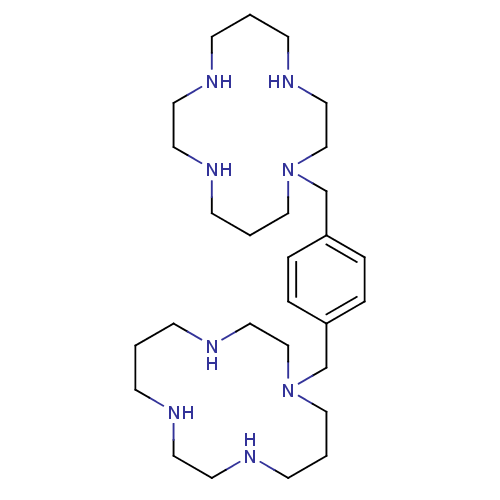 (1,1''-{1,4-phenylenebis(methylene)}-bis{1,4,8,11-t...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles Curated by ChEMBL | Assay Description Displacement of [125I]SDF1alpha from CCR2/CXCR4 (unknown origin) expressed in CHOK1 cells | J Biol Chem 282: 30062-9 (2007) Article DOI: 10.1074/jbc.M705302200 BindingDB Entry DOI: 10.7270/Q27M07Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50088301 ((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles Curated by ChEMBL | Assay Description Displacement of [125I]SDF1alpha from CCR2/CXCR4 (unknown origin) expressed in CHOK1 cells | J Biol Chem 282: 30062-9 (2007) Article DOI: 10.1074/jbc.M705302200 BindingDB Entry DOI: 10.7270/Q27M07Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50035696 (1,1''-{1,4-phenylenebis(methylene)}-bis{1,4,8,11-t...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles Curated by ChEMBL | Assay Description Displacement of [125I]MCP1 from CCR2/CXCR4 (unknown origin) expressed in CHOK1 cells | J Biol Chem 282: 30062-9 (2007) Article DOI: 10.1074/jbc.M705302200 BindingDB Entry DOI: 10.7270/Q27M07Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50257994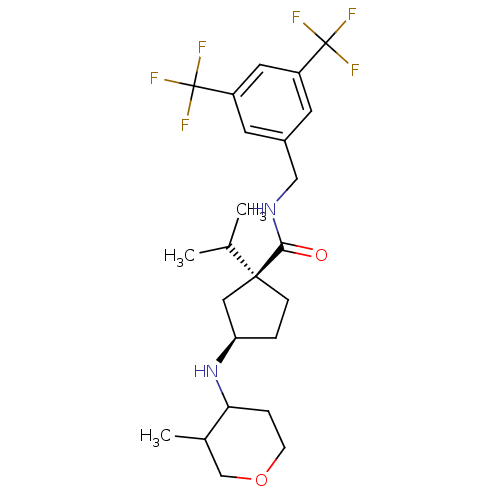 ((1S,3R)-N-(3,5-bis(-trifluoromethyl)benzyl)-1-iso ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor expressed in CHO cells by chemotaxis assay | Bioorg Med Chem Lett 19: 1830-4 (2009) Article DOI: 10.1016/j.bmcl.2008.12.050 BindingDB Entry DOI: 10.7270/Q2GH9HVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50257994 ((1S,3R)-N-(3,5-bis(-trifluoromethyl)benzyl)-1-iso ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor expressed in CHO cells by chemotaxis assay | Bioorg Med Chem Lett 19: 1830-4 (2009) Article DOI: 10.1016/j.bmcl.2008.12.050 BindingDB Entry DOI: 10.7270/Q2GH9HVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50257994 ((1S,3R)-N-(3,5-bis(-trifluoromethyl)benzyl)-1-iso ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor expressed in CHO cells by chemotaxis assay | Bioorg Med Chem Lett 19: 1830-4 (2009) Article DOI: 10.1016/j.bmcl.2008.12.050 BindingDB Entry DOI: 10.7270/Q2GH9HVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50212136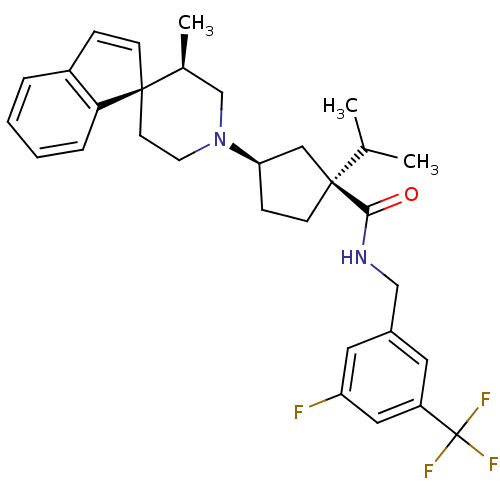 ((1S,3R)-N-{[3-fluoro-5-(trifluoromethyl)phenyl]met...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of chemotaxis in human MCP1 monocytes | Bioorg Med Chem Lett 17: 3636-41 (2007) Article DOI: 10.1016/j.bmcl.2007.04.053 BindingDB Entry DOI: 10.7270/Q23J3CN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50009224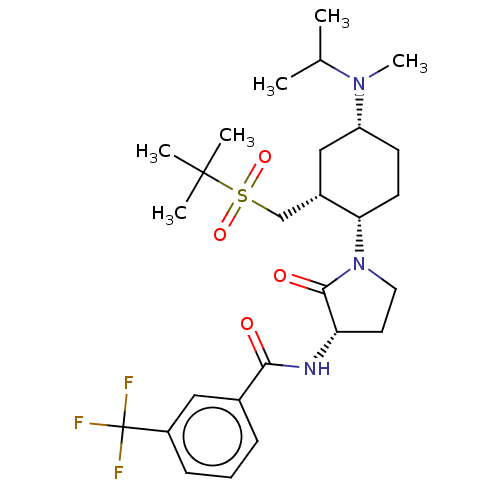 (CHEMBL3233188) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human monocytes assessed as reduction of chemotaxis in presence of 0.1 M BSA | Bioorg Med Chem Lett 24: 1843-5 (2014) Article DOI: 10.1016/j.bmcl.2014.02.013 BindingDB Entry DOI: 10.7270/Q2FQ9Z4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50009220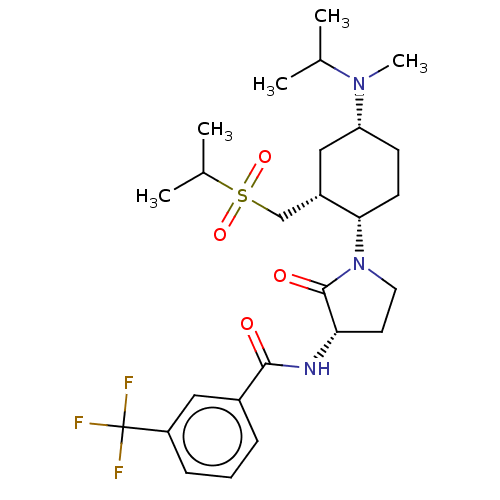 (CHEMBL3233186) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human monocytes assessed as reduction of chemotaxis in presence of 0.1 M BSA | Bioorg Med Chem Lett 24: 1843-5 (2014) Article DOI: 10.1016/j.bmcl.2014.02.013 BindingDB Entry DOI: 10.7270/Q2FQ9Z4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50268371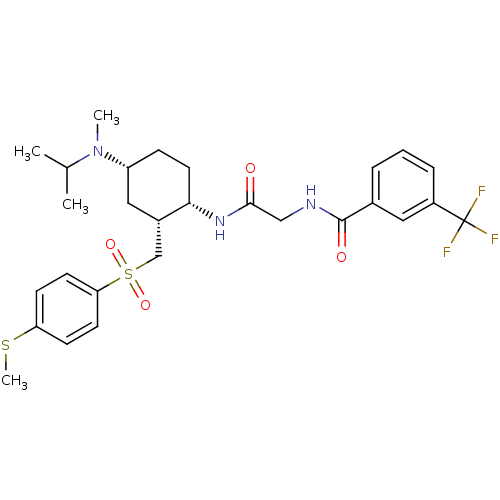 (CHEMBL502247 | N-(2-((1S,2R,4R)-4-(isopropyl(methy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human PBMC assessed as MCP1-induced chemotaxis | Bioorg Med Chem Lett 19: 3418-22 (2009) Article DOI: 10.1016/j.bmcl.2009.05.041 BindingDB Entry DOI: 10.7270/Q27W6C3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50377038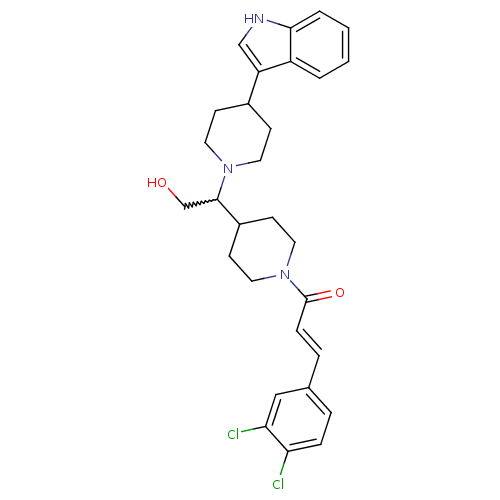 (CHEMBL258205) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity at human CCR2 receptor | Bioorg Med Chem Lett 18: 3562-4 (2008) Article DOI: 10.1016/j.bmcl.2008.05.010 BindingDB Entry DOI: 10.7270/Q2Q52QGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50212127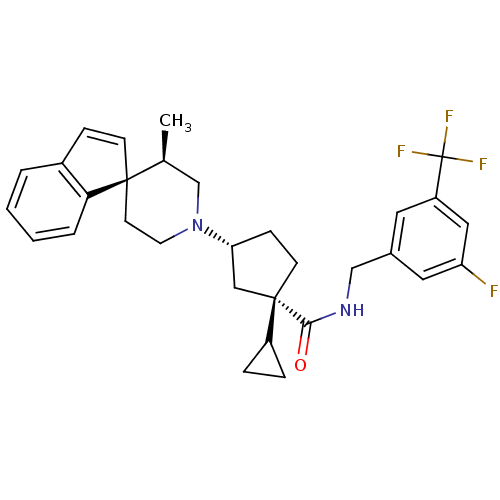 ((1S,3R)-1-cyclopropyl-N-{[3-fluoro-5-(trifluoromet...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of chemotaxis in human MCP1 monocytes | Bioorg Med Chem Lett 17: 3636-41 (2007) Article DOI: 10.1016/j.bmcl.2007.04.053 BindingDB Entry DOI: 10.7270/Q23J3CN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089354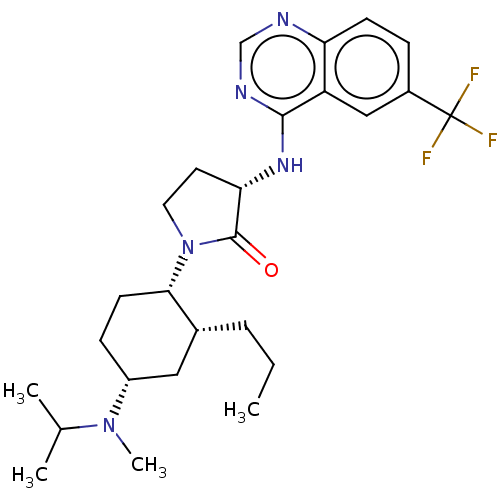 (CHEMBL3577945) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity against CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis at 37 degC | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50088301 ((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Libre de Bruxelles Curated by ChEMBL | Assay Description Displacement of [125I]MCP1 from CCR2 (unknown origin) expressed in CHOK1 cells | J Biol Chem 282: 30062-9 (2007) Article DOI: 10.1074/jbc.M705302200 BindingDB Entry DOI: 10.7270/Q27M07Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50012008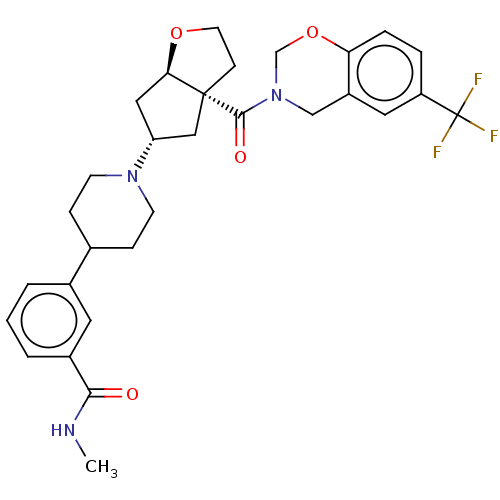 (CHEMBL3263286) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, LLC Curated by ChEMBL | Assay Description Displacement of labeled MCP-1 from human CCR2 expressed in THP1 cells | Bioorg Med Chem Lett 24: 2137-40 (2014) Article DOI: 10.1016/j.bmcl.2014.03.036 BindingDB Entry DOI: 10.7270/Q2TH8P7P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50268423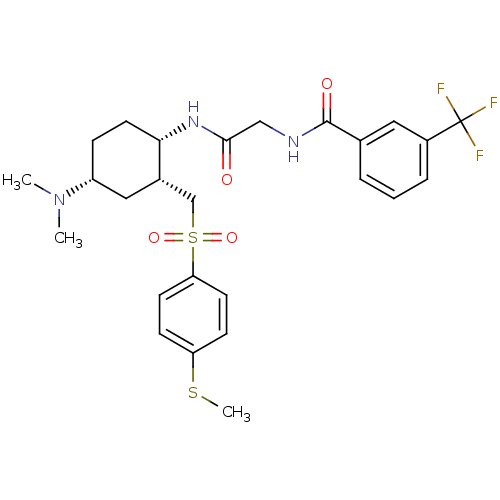 (CHEMBL497202 | N-(2-((1S,2R,4R)-4-(dimethylamino)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human PBMC assessed as MCP1-induced calcium flux by fluorescence-imaging plate reader assay | Bioorg Med Chem Lett 19: 3418-22 (2009) Article DOI: 10.1016/j.bmcl.2009.05.041 BindingDB Entry DOI: 10.7270/Q27W6C3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50315998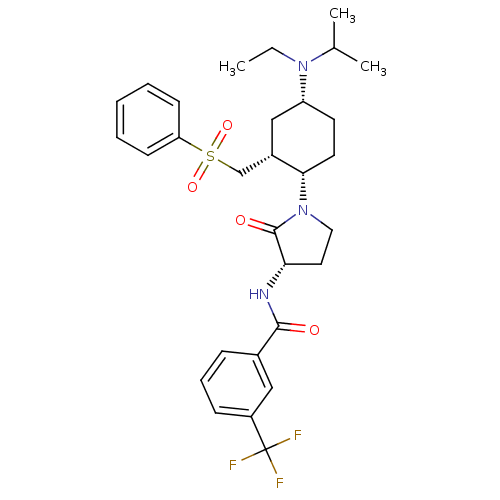 (CHEMBL1090893 | N-((S)-1-((1S,2R,4R)-4-(ethyl(isop...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]-MCP1 from CCR2 in human THP1 cells after 30 mins | Bioorg Med Chem Lett 20: 2425-30 (2010) Article DOI: 10.1016/j.bmcl.2010.03.035 BindingDB Entry DOI: 10.7270/Q2ZK5GTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50557876 (CHEMBL4764460) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as reduction in MCP1-induced chemotaxis measured after 30 mins by calcein-AM dye based fluor... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00082 BindingDB Entry DOI: 10.7270/Q24J0JS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50257995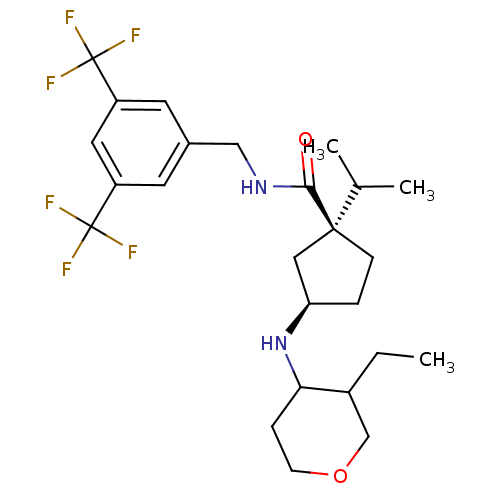 ((1S,3R)-N-(3,5-bis(trifluoromethyl)benzyl)-3-(3-et...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor expressed in CHO cells by chemotaxis assay | Bioorg Med Chem Lett 19: 1830-4 (2009) Article DOI: 10.1016/j.bmcl.2008.12.050 BindingDB Entry DOI: 10.7270/Q2GH9HVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50212128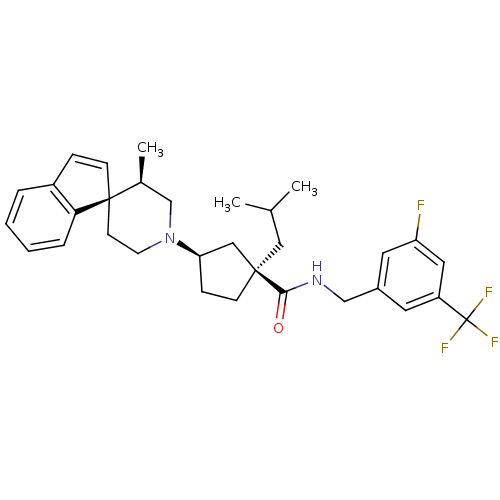 ((1R,3R)-N-{[3-fluoro-5-(trifluoromethyl)phenyl]met...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of chemotaxis in human MCP1 monocytes | Bioorg Med Chem Lett 17: 3636-41 (2007) Article DOI: 10.1016/j.bmcl.2007.04.053 BindingDB Entry DOI: 10.7270/Q23J3CN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50009220 (CHEMBL3233186) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]-labeled human MCP1 from CCR2 in human PBMC | Bioorg Med Chem Lett 24: 1843-5 (2014) Article DOI: 10.1016/j.bmcl.2014.02.013 BindingDB Entry DOI: 10.7270/Q2FQ9Z4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50268423 (CHEMBL497202 | N-(2-((1S,2R,4R)-4-(dimethylamino)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]MCP1 from CCR2 in human PBMC by millipore filter plate assay | Bioorg Med Chem Lett 19: 3418-22 (2009) Article DOI: 10.1016/j.bmcl.2009.05.041 BindingDB Entry DOI: 10.7270/Q27W6C3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089366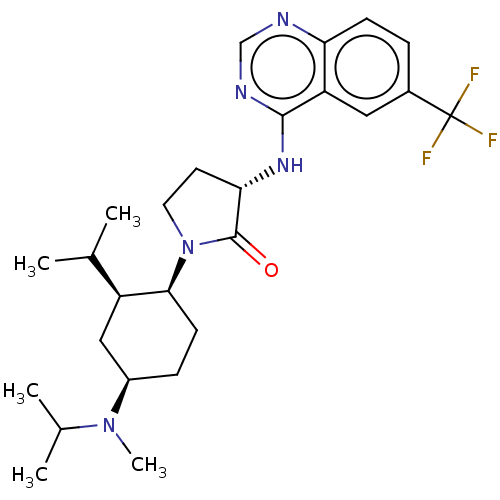 (CHEMBL3577948) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of [125I]hMCP1 binding to CCR2 in human PBMC incubated for 30 mins at room temperature | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50315992 (CHEMBL1092678 | N-((S)-1-((1S,2R,4R)-4-(isopropyl(...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]-MCP1 from CCR2 in human THP1 cells after 30 mins | Bioorg Med Chem Lett 20: 2425-30 (2010) Article DOI: 10.1016/j.bmcl.2010.03.035 BindingDB Entry DOI: 10.7270/Q2ZK5GTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50509862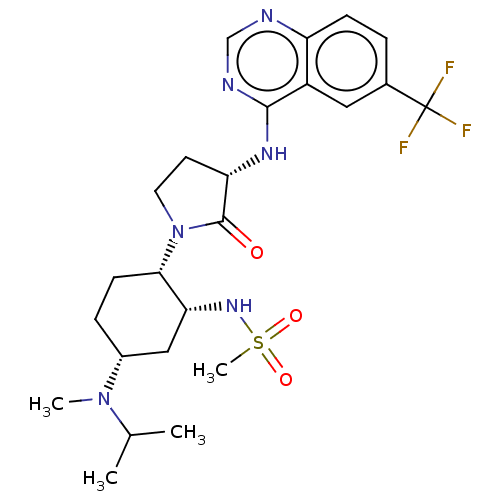 (CHEMBL4457723) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]MCP1 from CCR2 in human PBMC measured after 30 mins | ACS Med Chem Lett 10: 300-305 (2019) Article DOI: 10.1021/acsmedchemlett.8b00439 BindingDB Entry DOI: 10.7270/Q22R3W0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50198149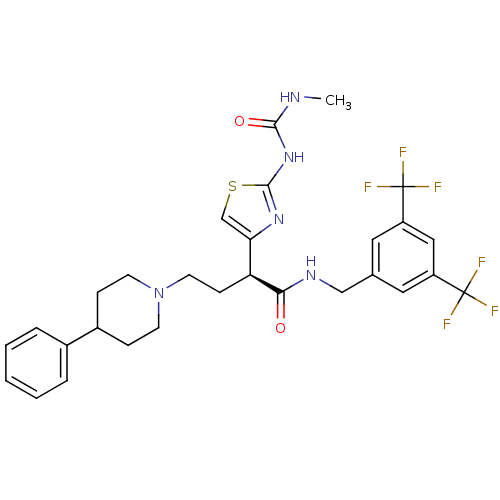 ((S)-1-(4-(1-(3,5-bis(trifluoromethyl)benzylamino)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]MCP1 from human CCR2 expressed in monocytes | Bioorg Med Chem Lett 17: 309-14 (2007) Article DOI: 10.1016/j.bmcl.2006.10.059 BindingDB Entry DOI: 10.7270/Q25B0247 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50198149 ((S)-1-(4-(1-(3,5-bis(trifluoromethyl)benzylamino)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2b receptor | Bioorg Med Chem Lett 18: 1323-30 (2008) Article DOI: 10.1016/j.bmcl.2008.01.023 BindingDB Entry DOI: 10.7270/Q2SN08Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089355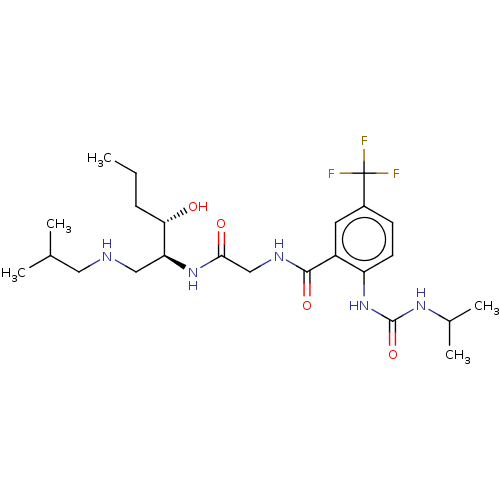 (CHEMBL3577932) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity against CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis at 37 degC | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089356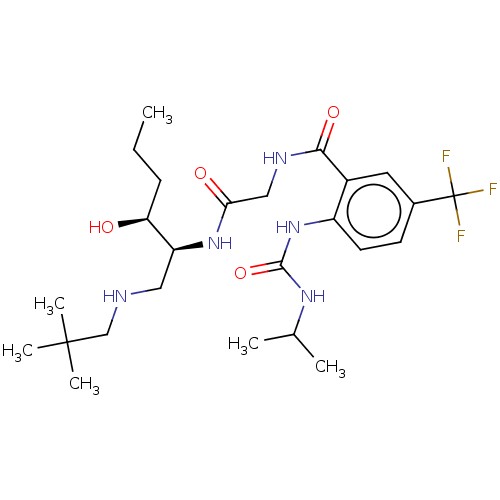 (CHEMBL3577933) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity against CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis at 37 degC | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50009221 (CHEMBL3233187) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]-labeled human MCP1 from CCR2 in human PBMC | Bioorg Med Chem Lett 24: 1843-5 (2014) Article DOI: 10.1016/j.bmcl.2014.02.013 BindingDB Entry DOI: 10.7270/Q2FQ9Z4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50315993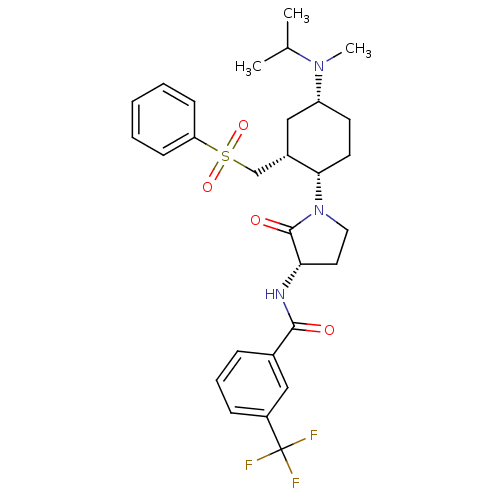 (CHEMBL1091605 | N-((S)-1-((1S,2R,4R)-4-(isopropyl(...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis after 45 mins in presence of 0.1 M bovine serum albumin | Bioorg Med Chem Lett 20: 2425-30 (2010) Article DOI: 10.1016/j.bmcl.2010.03.035 BindingDB Entry DOI: 10.7270/Q2ZK5GTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50371414 (CHEMBL271828) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 in PBMCs assessed as inhibition of chemotaxis | J Med Chem 51: 721-4 (2008) Article DOI: 10.1021/jm701488f BindingDB Entry DOI: 10.7270/Q2TX3G60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50268371 (CHEMBL502247 | N-(2-((1S,2R,4R)-4-(isopropyl(methy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human PBMC assessed as MCP1-induced calcium flux by fluorescence-imaging plate reader assay | Bioorg Med Chem Lett 19: 3418-22 (2009) Article DOI: 10.1016/j.bmcl.2009.05.041 BindingDB Entry DOI: 10.7270/Q27W6C3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50315993 (CHEMBL1091605 | N-((S)-1-((1S,2R,4R)-4-(isopropyl(...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human monocytes assessed as reduction of chemotaxis in presence of 0.1 M BSA | Bioorg Med Chem Lett 24: 1843-5 (2014) Article DOI: 10.1016/j.bmcl.2014.02.013 BindingDB Entry DOI: 10.7270/Q2FQ9Z4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089364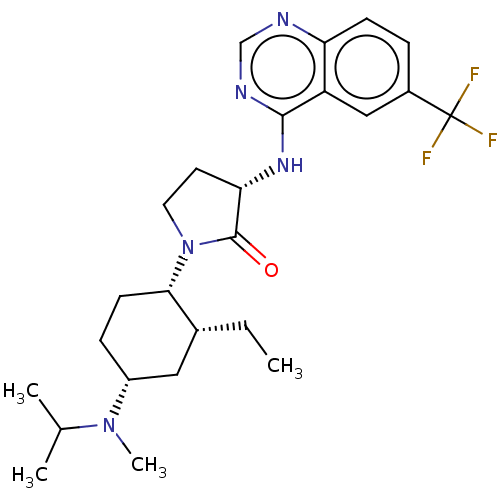 (CHEMBL3577946) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of [125I]hMCP1 binding to CCR2 in human PBMC incubated for 30 mins at room temperature | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089364 (CHEMBL3577946) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity against CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis at 37 degC | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50382932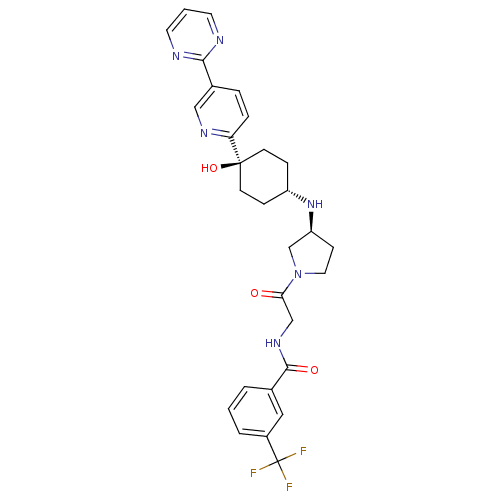 (CHEMBL2029422) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of CCR2-mediated Erk phosphorylation | ACS Med Chem Lett 2: 913-918 (2011) Article DOI: 10.1021/ml200199c BindingDB Entry DOI: 10.7270/Q29024TK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50377034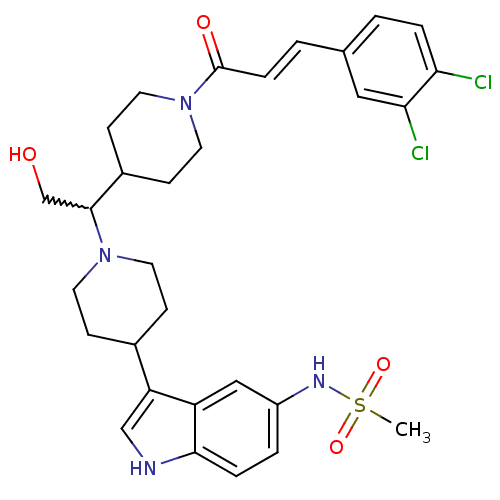 (CHEMBL256301) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity at human CCR2 receptor | Bioorg Med Chem Lett 18: 3562-4 (2008) Article DOI: 10.1016/j.bmcl.2008.05.010 BindingDB Entry DOI: 10.7270/Q2Q52QGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50198115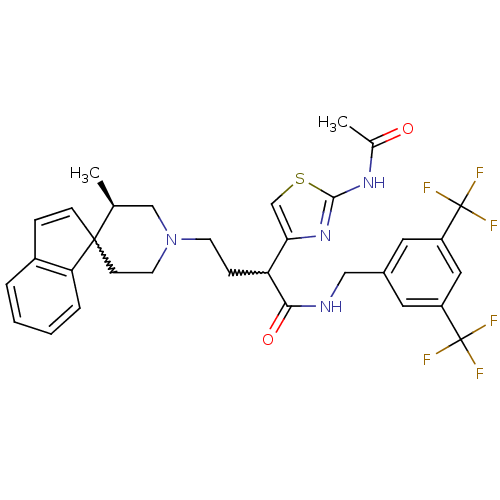 (CHEMBL438304 | N-{[3,5-bis(trifluoromethyl)phenyl]...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of MCP1-stimulated chemotaxis in monocytes transfected with human CCR2 | Bioorg Med Chem Lett 17: 309-14 (2007) Article DOI: 10.1016/j.bmcl.2006.10.059 BindingDB Entry DOI: 10.7270/Q25B0247 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089353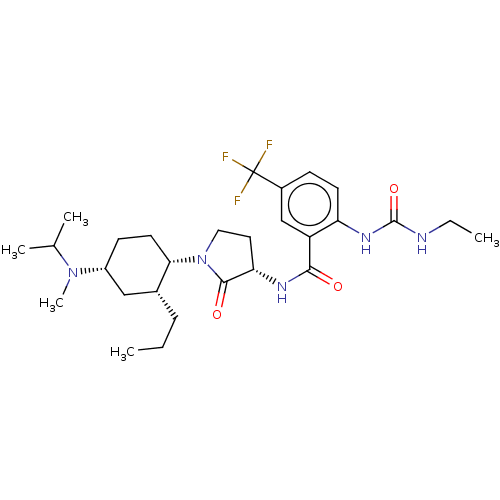 (CHEMBL3577942) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity against CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis at 37 degC | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50009217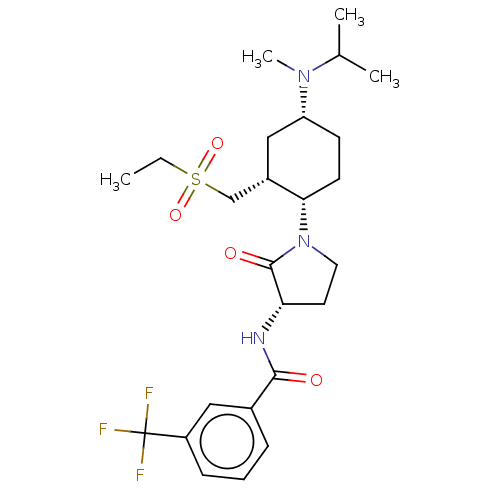 (CHEMBL3233184) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity at CCR2 in human monocytes assessed as reduction of chemotaxis in presence of 0.1 M BSA | Bioorg Med Chem Lett 24: 1843-5 (2014) Article DOI: 10.1016/j.bmcl.2014.02.013 BindingDB Entry DOI: 10.7270/Q2FQ9Z4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50557877 (CHEMBL4790208) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as reduction in MCP1-induced chemotaxis measured after 30 mins by calcein-AM dye based fluor... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00082 BindingDB Entry DOI: 10.7270/Q24J0JS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50377039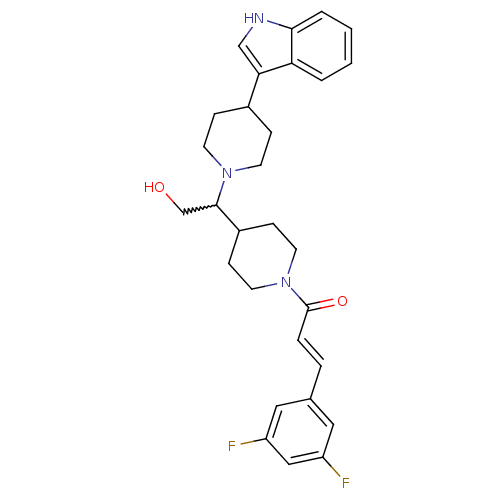 (CHEMBL257191) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity at human CCR2 receptor | Bioorg Med Chem Lett 18: 3562-4 (2008) Article DOI: 10.1016/j.bmcl.2008.05.010 BindingDB Entry DOI: 10.7270/Q2Q52QGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50212127 ((1S,3R)-1-cyclopropyl-N-{[3-fluoro-5-(trifluoromet...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Ca2+ flux in human monocytes | Bioorg Med Chem Lett 17: 3636-41 (2007) Article DOI: 10.1016/j.bmcl.2007.04.053 BindingDB Entry DOI: 10.7270/Q23J3CN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50509860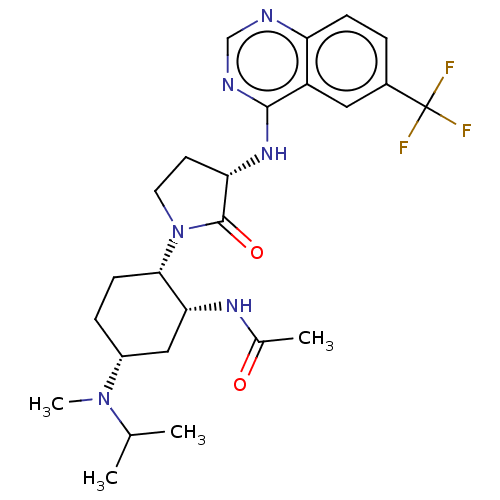 (CHEMBL4442783) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of CCR2 in human THP1 cells assessed as reduction in MCP1-induced chemotaxis measured after 10 mins by calcein-AM dye based fluorescence a... | ACS Med Chem Lett 10: 300-305 (2019) Article DOI: 10.1021/acsmedchemlett.8b00439 BindingDB Entry DOI: 10.7270/Q22R3W0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089354 (CHEMBL3577945) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]MCP1 from CCR2 in human PBMC measured after 30 mins | ACS Med Chem Lett 10: 300-305 (2019) Article DOI: 10.1021/acsmedchemlett.8b00439 BindingDB Entry DOI: 10.7270/Q22R3W0Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50509860 (CHEMBL4442783) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as reduction in MCP1-induced chemotaxis measured after 30 mins by calcein-AM dye based fluor... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00082 BindingDB Entry DOI: 10.7270/Q24J0JS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50509860 (CHEMBL4442783) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CCR2 in human THP-1 cells assessed as reduction in CCL2-induced chemotaxis | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00373 BindingDB Entry DOI: 10.7270/Q2M04981 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50009214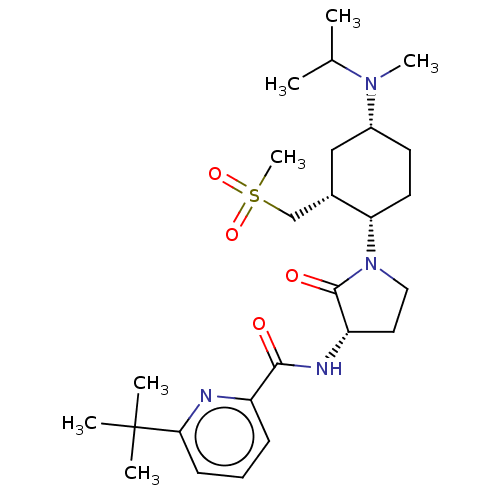 (CHEMBL3233181) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]-labeled human MCP1 from CCR2 in human PBMC | Bioorg Med Chem Lett 24: 1843-5 (2014) Article DOI: 10.1016/j.bmcl.2014.02.013 BindingDB Entry DOI: 10.7270/Q2FQ9Z4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3307 total ) | Next | Last >> |