

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C Virus) | BDBM50495931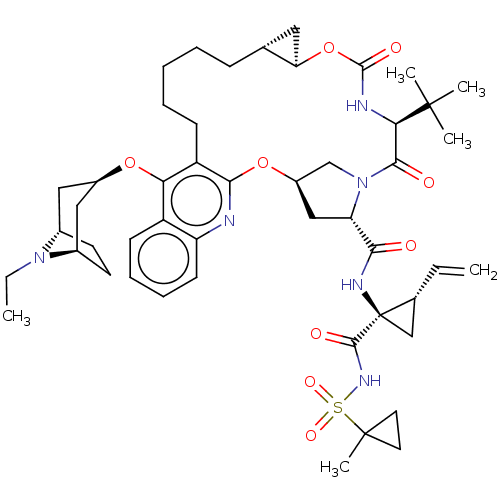 (CHEMBL3348818) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease R155K mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495935 (CHEMBL3120475) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease R155K mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495927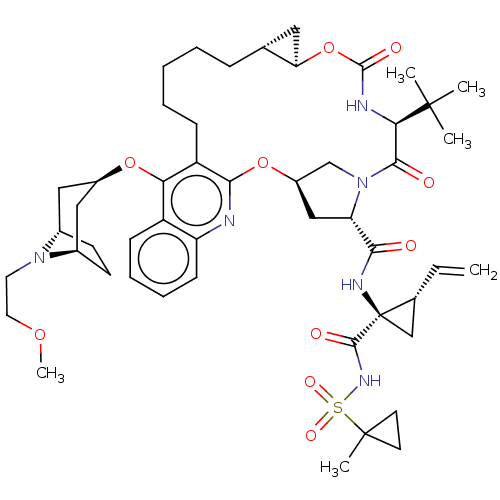 (CHEMBL3348817) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease R155K mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495928 (CHEMBL3349199) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease R155K mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495924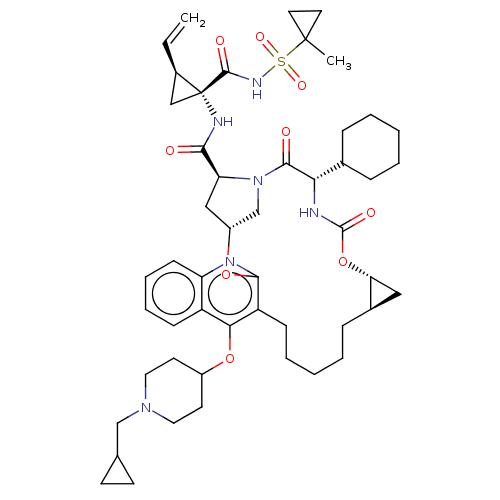 (CHEMBL3120477) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease R155K mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495931 (CHEMBL3348818) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease D168Y mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495936 (CHEMBL3120492) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease R155K mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495943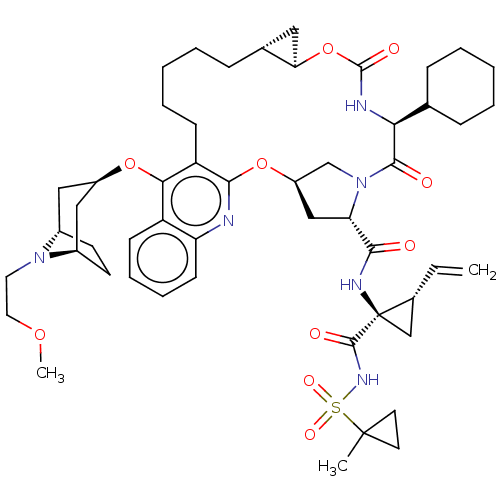 (CHEMBL3349202) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease R155K mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495928 (CHEMBL3349199) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease D168Y mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495927 (CHEMBL3348817) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease D168Y mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495929 (CHEMBL3120493) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease R155K mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50485492 (Grazoprevir | Grazoprevir monohydrate | MK-5172 | ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease R155K mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495928 (CHEMBL3349199) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease A156T mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495935 (CHEMBL3120475) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease D168Y mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495941 (CHEMBL3349203) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease R155K mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495942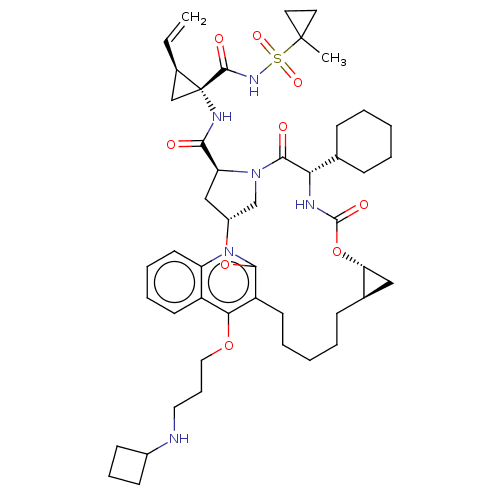 (CHEMBL3120488) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease R155K mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495924 (CHEMBL3120477) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease D168Y mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495934 (CHEMBL3120476) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease R155K mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495932 (CHEMBL3349201) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease R155K mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495928 (CHEMBL3349199) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease A156V mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495926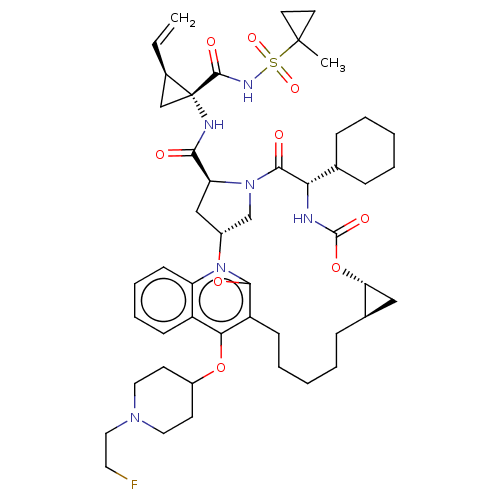 (CHEMBL3120482) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease R155K mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50485492 (Grazoprevir | Grazoprevir monohydrate | MK-5172 | ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease D168Y mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495945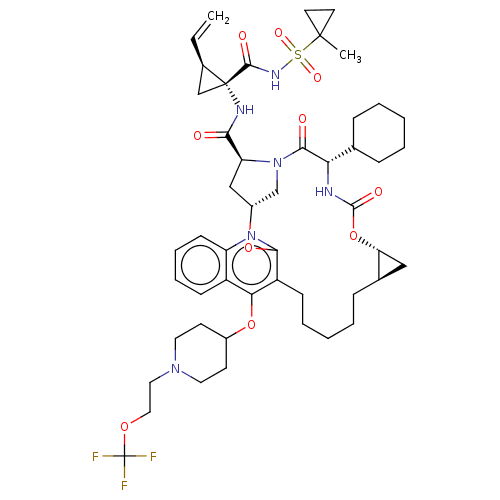 (CHEMBL3120479) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease R155K mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495925 (CHEMBL3120478) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease D168Y mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495938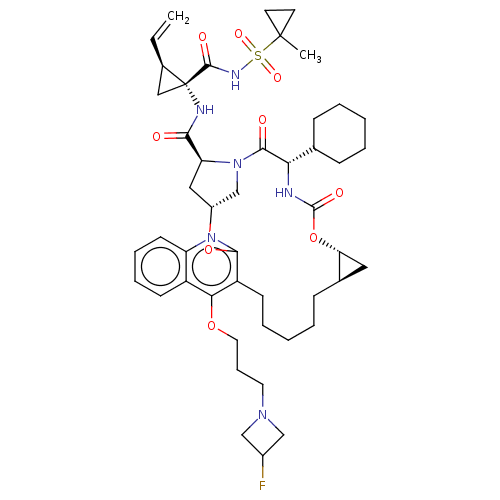 (CHEMBL3120489) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease R155K mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495925 (CHEMBL3120478) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease R155K mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495939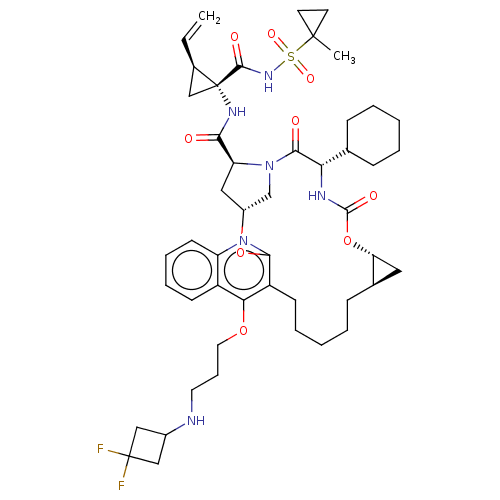 (CHEMBL3120169) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease R155K mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495943 (CHEMBL3349202) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease D168Y mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495931 (CHEMBL3348818) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease A156T mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495934 (CHEMBL3120476) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease D168Y mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495940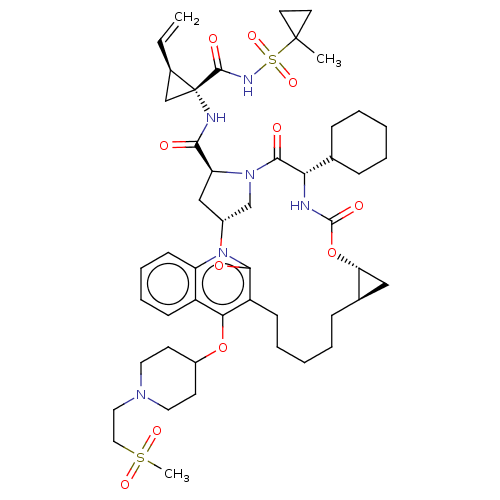 (CHEMBL3120480) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease R155K mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495935 (CHEMBL3120475) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease A156T mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM103840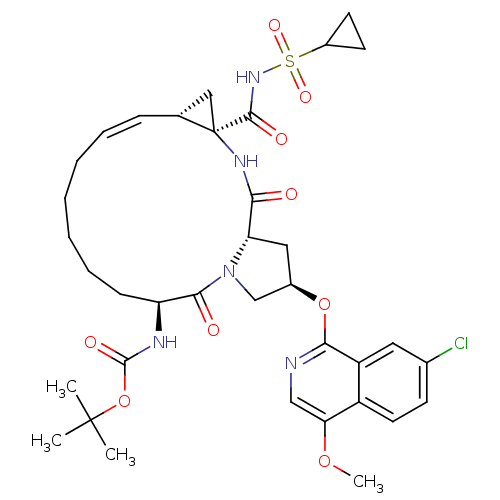 (Asu-mcP1P3) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School | Assay Description NS3/4A protease was preincubated with increasing concentration of drugs in protease reaction buffer for 1 hour. Inhibition assays were performed in ... | ACS Chem Biol 8: 1469-78 (2013) Article DOI: 10.1021/cb400100g BindingDB Entry DOI: 10.7270/Q2FQ9V7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM103839 (Danoprevir) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School | Assay Description NS3/4A protease was preincubated with increasing concentration of drugs in protease reaction buffer for 1 hour. Inhibition assays were performed in ... | ACS Chem Biol 8: 1469-78 (2013) Article DOI: 10.1021/cb400100g BindingDB Entry DOI: 10.7270/Q2FQ9V7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495935 (CHEMBL3120475) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease A156V mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495933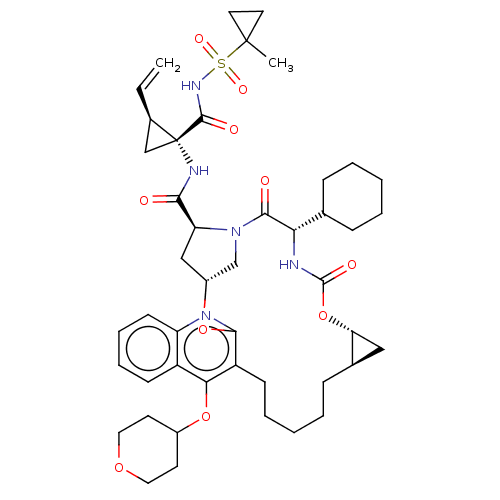 (CHEMBL3120483) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease R155K mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM103841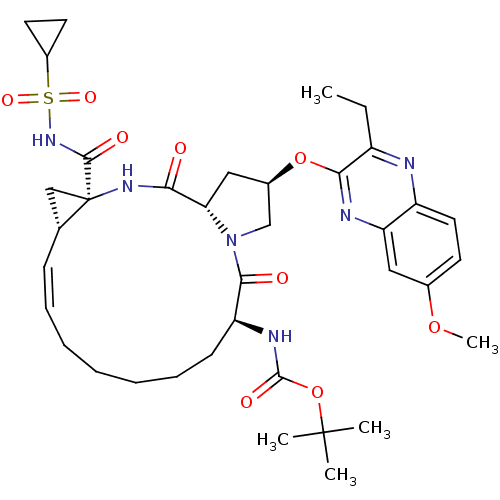 (5172-mcP1P3) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School | Assay Description NS3/4A protease was preincubated with increasing concentration of drugs in protease reaction buffer for 1 hour. Inhibition assays were performed in ... | ACS Chem Biol 8: 1469-78 (2013) Article DOI: 10.1021/cb400100g BindingDB Entry DOI: 10.7270/Q2FQ9V7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495936 (CHEMBL3120492) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease A156V mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495924 (CHEMBL3120477) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease A156V mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495941 (CHEMBL3349203) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease D168Y mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495937 (CHEMBL3120491) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease R155K mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495944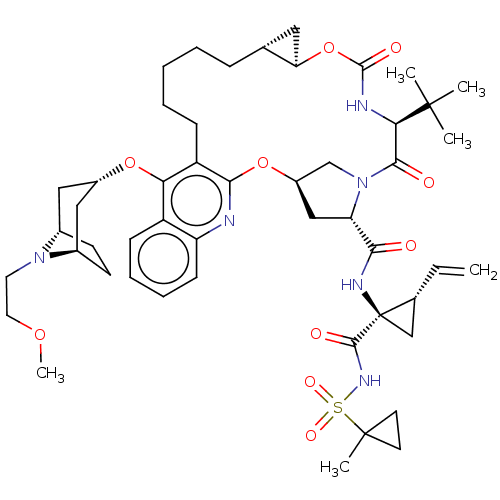 (CHEMBL3349200) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease R155K mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495929 (CHEMBL3120493) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease D168Y mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM103838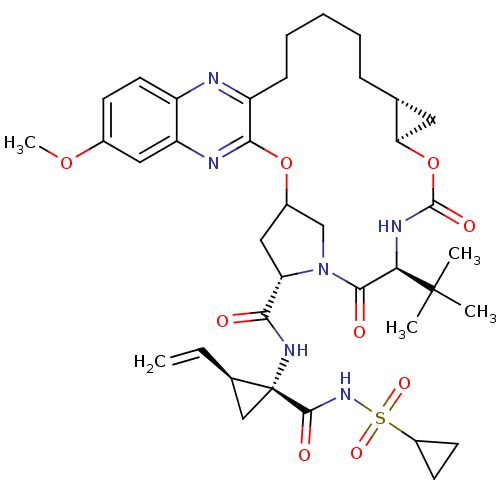 (MK-5172) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School | Assay Description NS3/4A protease was preincubated with increasing concentration of drugs in protease reaction buffer for 1 hour. Inhibition assays were performed in ... | ACS Chem Biol 8: 1469-78 (2013) Article DOI: 10.1021/cb400100g BindingDB Entry DOI: 10.7270/Q2FQ9V7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM196124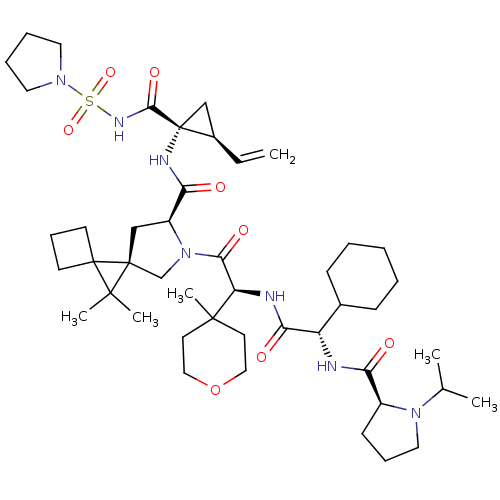 (US9206232, 125) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds of Table A against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length ... | US Patent US9206232 (2015) BindingDB Entry DOI: 10.7270/Q2M0447H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM110357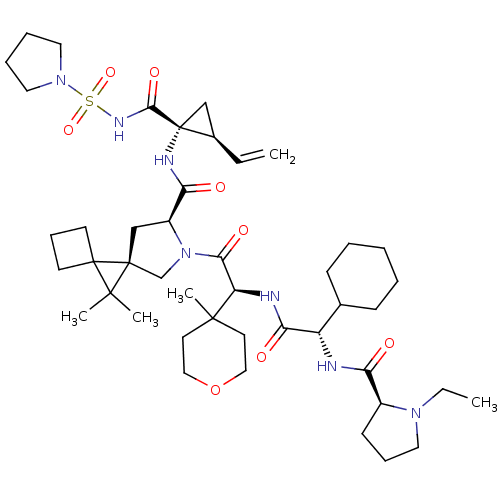 (US8613914, 125 | US9206232, 129) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of certain compounds against HCV NS3-4A serine protease is determined in a homogenous assay using the full-length NS3-4A prot... | US Patent US8613914 (2013) BindingDB Entry DOI: 10.7270/Q28C9TWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495942 (CHEMBL3120488) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease A156V mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495942 (CHEMBL3120488) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease D168Y mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495939 (CHEMBL3120169) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease D168Y mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM50495931 (CHEMBL3348818) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus genotype 1b NS3/4A protease A156V mutant assessed as substrate cleavage using Ac-C(Eu)DDMEEAbu(COO)ASK(QSY7)-amide as... | ACS Med Chem Lett 5: 264-9 (2014) Article DOI: 10.1021/ml400466p BindingDB Entry DOI: 10.7270/Q22R3VNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3539 total ) | Next | Last >> |