

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50191945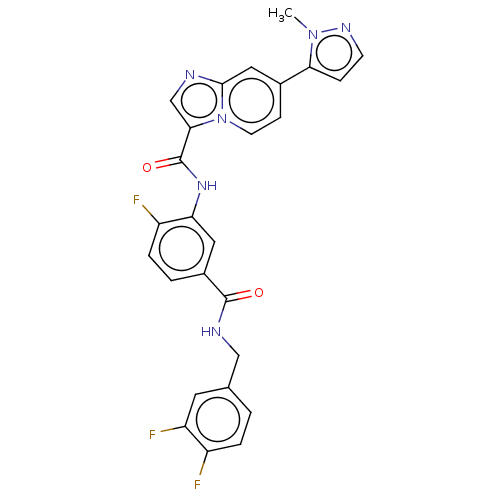 (CHEMBL3904768) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50191977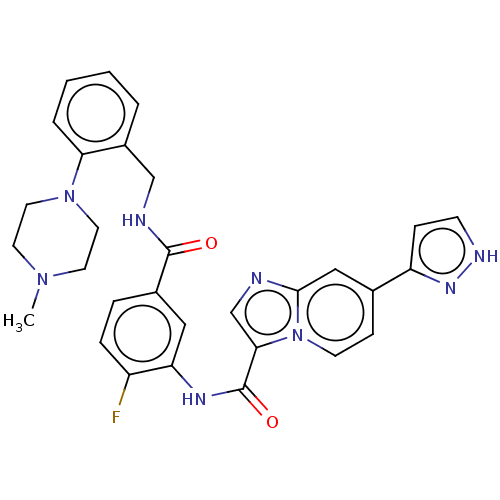 (CHEMBL3983564) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha | J Med Chem 52: 3191-204 (2009) Article DOI: 10.1021/jm800861c BindingDB Entry DOI: 10.7270/Q23J3DWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50265746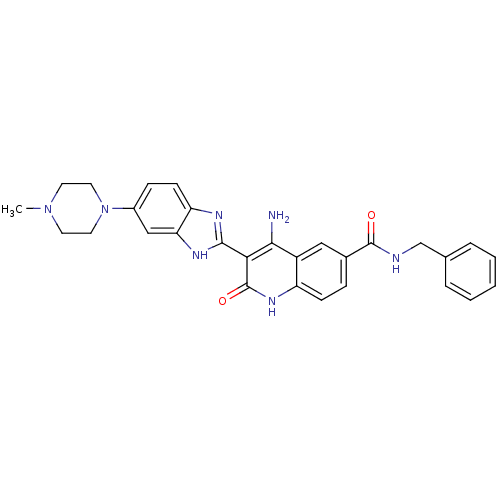 (CHEMBL526507 | {4-Amino-3-[6-(4-methylpiperazinyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant PDGFRbeta | J Med Chem 52: 278-92 (2009) Article DOI: 10.1021/jm800790t BindingDB Entry DOI: 10.7270/Q2TD9X7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50265774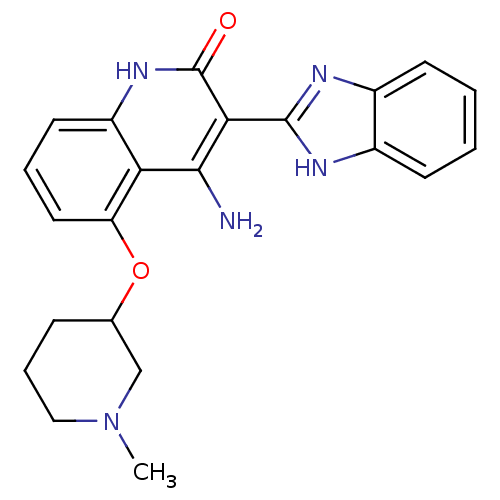 (4-Amino-3-benzimidazol-2-yl-5-(1-methyl(3-piperidy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant PDGFRbeta | J Med Chem 52: 278-92 (2009) Article DOI: 10.1021/jm800790t BindingDB Entry DOI: 10.7270/Q2TD9X7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM581371 (US11505527, Compound 4j) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description An in vitro kinase assay was performed to evaluate the kinase suppression activity of the most promising cytotoxic candidates 4b, 4j against four dif... | Citation and Details BindingDB Entry DOI: 10.7270/Q25B06BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50191981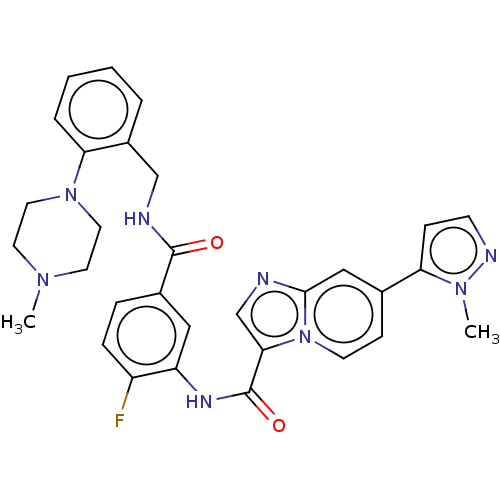 (CHEMBL3979322) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50191973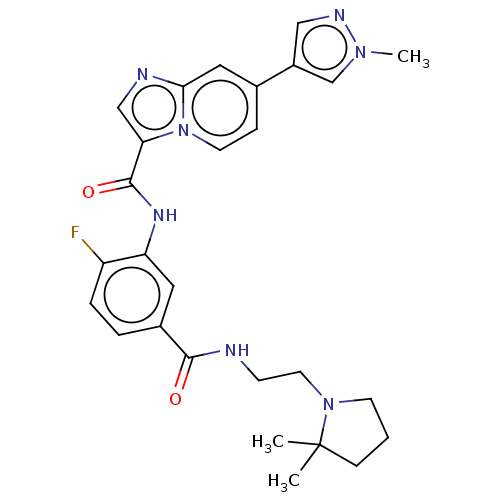 (CHEMBL3940697) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50381946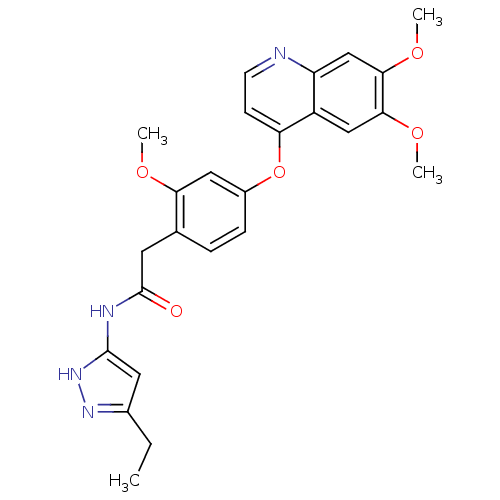 (CHEMBL2023485) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of PDGFRbeta | Bioorg Med Chem Lett 22: 3050-5 (2012) Article DOI: 10.1016/j.bmcl.2012.03.074 BindingDB Entry DOI: 10.7270/Q298881Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50381945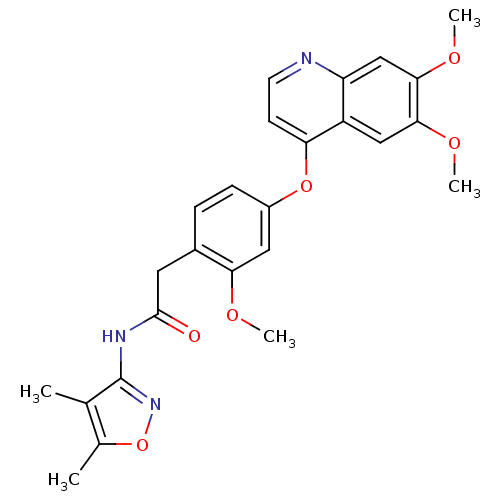 (CHEMBL2023477) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of PDGFRbeta | Bioorg Med Chem Lett 22: 3050-5 (2012) Article DOI: 10.1016/j.bmcl.2012.03.074 BindingDB Entry DOI: 10.7270/Q298881Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50191978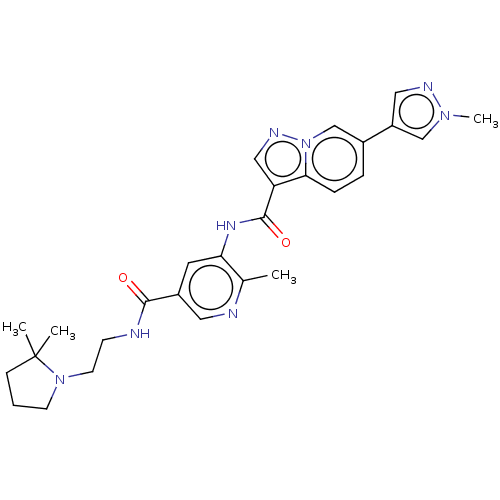 (CHEMBL3915941) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50265574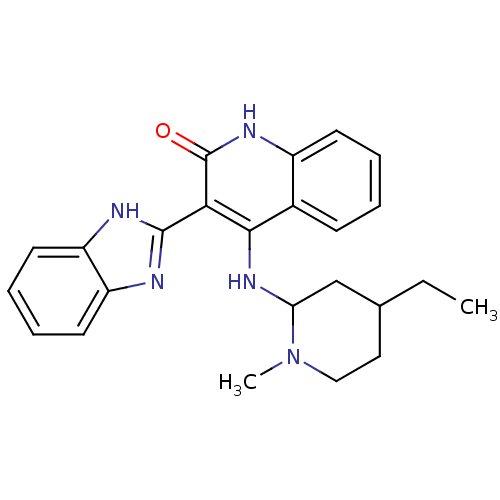 (3-(1H-benzo[d]imidazol-2-yl)-4-(4-ethyl-1-methylpi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant PDGFRbeta | J Med Chem 52: 278-92 (2009) Article DOI: 10.1021/jm800790t BindingDB Entry DOI: 10.7270/Q2TD9X7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50336457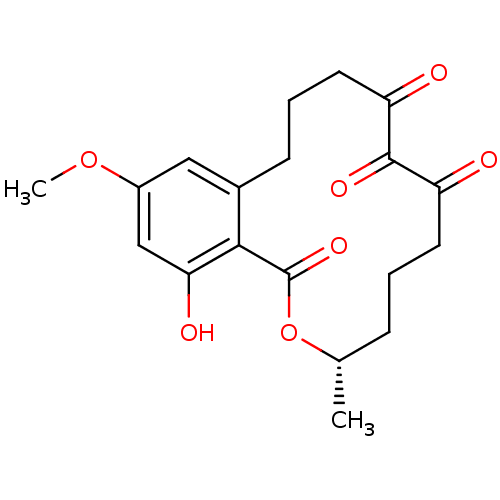 ((3S,5Z,8S,9R,11E)-8,9,16-trihydroxy-14-methoxy-3-m...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Curated by ChEMBL | Assay Description Inhibition of human recombinant PDGFRalpha in cell free system after 60 mins | Bioorg Med Chem Lett 21: 1167-70 (2011) Article DOI: 10.1016/j.bmcl.2010.12.100 BindingDB Entry DOI: 10.7270/Q23T9HH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM16673 (4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank US Patent | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description An in vitro kinase assay was performed to evaluate the kinase suppression activity of the most promising cytotoxic candidates 4b, 4j against four dif... | Citation and Details BindingDB Entry DOI: 10.7270/Q25B06BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha [D842V] (Homo sapiens (Human)) | BDBM472776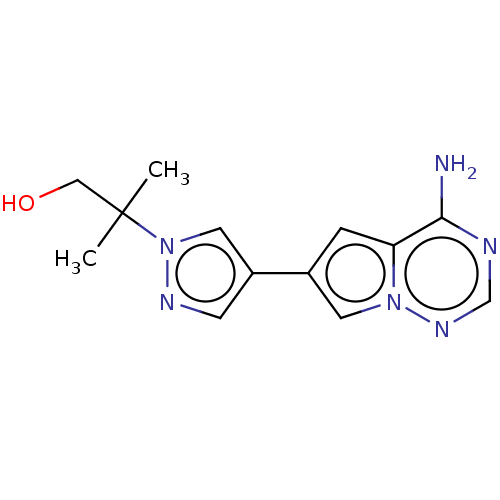 (US10829493, Example 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Blueprint Medicines Corporation US Patent | Assay Description KIT D816V assay at the APPKM for ATP: In each well of a 384-well assay plate, 0.3 nM of untreated enzyme was incubated in a total of 13 μL of bu... | US Patent US10829493 (2020) BindingDB Entry DOI: 10.7270/Q2MP56BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha [D842V] (Homo sapiens (Human)) | BDBM472777 (US10829493, Example 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Blueprint Medicines Corporation US Patent | Assay Description KIT D816V assay at the APPKM for ATP: In each well of a 384-well assay plate, 0.3 nM of untreated enzyme was incubated in a total of 13 μL of bu... | US Patent US10829493 (2020) BindingDB Entry DOI: 10.7270/Q2MP56BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha [D842V] (Homo sapiens (Human)) | BDBM472778 (US10829493, Example 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Blueprint Medicines Corporation US Patent | Assay Description KIT D816V assay at the APPKM for ATP: In each well of a 384-well assay plate, 0.3 nM of untreated enzyme was incubated in a total of 13 μL of bu... | US Patent US10829493 (2020) BindingDB Entry DOI: 10.7270/Q2MP56BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha [D842V] (Homo sapiens (Human)) | BDBM472779 (US10829493, Example 5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Blueprint Medicines Corporation US Patent | Assay Description KIT D816V assay at the APPKM for ATP: In each well of a 384-well assay plate, 0.3 nM of untreated enzyme was incubated in a total of 13 μL of bu... | US Patent US10829493 (2020) BindingDB Entry DOI: 10.7270/Q2MP56BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha [D842V] (Homo sapiens (Human)) | BDBM472780 (US10829493, Example 6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Blueprint Medicines Corporation US Patent | Assay Description KIT D816V assay at the APPKM for ATP: In each well of a 384-well assay plate, 0.3 nM of untreated enzyme was incubated in a total of 13 μL of bu... | US Patent US10829493 (2020) BindingDB Entry DOI: 10.7270/Q2MP56BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha [D842V] (Homo sapiens (Human)) | BDBM472781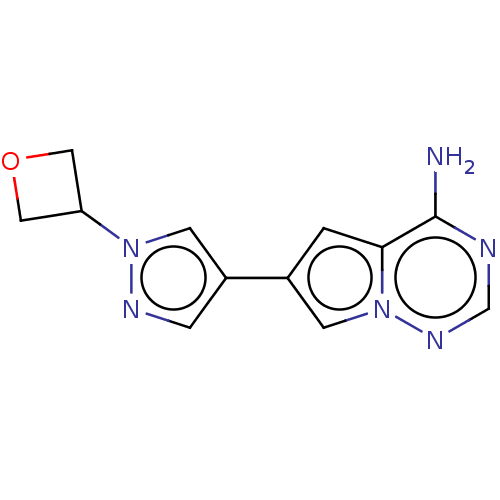 (US10829493, Example 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Blueprint Medicines Corporation US Patent | Assay Description KIT D816V assay at the APPKM for ATP: In each well of a 384-well assay plate, 0.3 nM of untreated enzyme was incubated in a total of 13 μL of bu... | US Patent US10829493 (2020) BindingDB Entry DOI: 10.7270/Q2MP56BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha [D842V] (Homo sapiens (Human)) | BDBM472782 (US10829493, Example 8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Blueprint Medicines Corporation US Patent | Assay Description KIT D816V assay at the APPKM for ATP: In each well of a 384-well assay plate, 0.3 nM of untreated enzyme was incubated in a total of 13 μL of bu... | US Patent US10829493 (2020) BindingDB Entry DOI: 10.7270/Q2MP56BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha [D842V] (Homo sapiens (Human)) | BDBM472783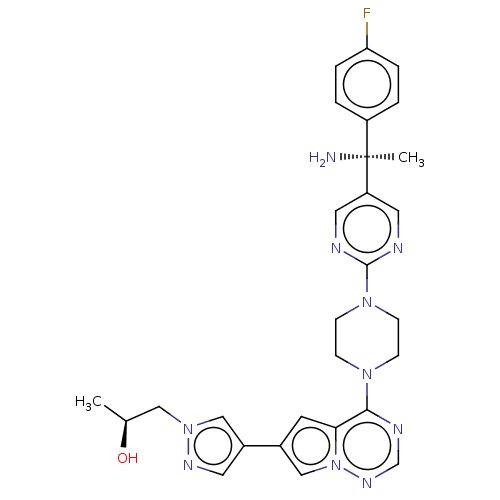 (US10829493, Example 9) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Blueprint Medicines Corporation US Patent | Assay Description KIT D816V assay at the APPKM for ATP: In each well of a 384-well assay plate, 0.3 nM of untreated enzyme was incubated in a total of 13 μL of bu... | US Patent US10829493 (2020) BindingDB Entry DOI: 10.7270/Q2MP56BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha [D842V] (Homo sapiens (Human)) | BDBM472784 (US10829493, Example 10) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Blueprint Medicines Corporation US Patent | Assay Description KIT D816V assay at the APPKM for ATP: In each well of a 384-well assay plate, 0.3 nM of untreated enzyme was incubated in a total of 13 μL of bu... | US Patent US10829493 (2020) BindingDB Entry DOI: 10.7270/Q2MP56BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha [D842V] (Homo sapiens (Human)) | BDBM472785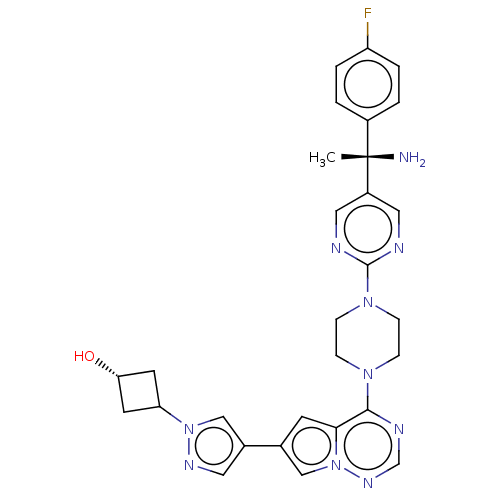 (US10829493, Example 11) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Blueprint Medicines Corporation US Patent | Assay Description KIT D816V assay at the APPKM for ATP: In each well of a 384-well assay plate, 0.3 nM of untreated enzyme was incubated in a total of 13 μL of bu... | US Patent US10829493 (2020) BindingDB Entry DOI: 10.7270/Q2MP56BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha [D842V] (Homo sapiens (Human)) | BDBM472786 (US10829493, Example 12) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Blueprint Medicines Corporation US Patent | Assay Description KIT D816V assay at the APPKM for ATP: In each well of a 384-well assay plate, 0.3 nM of untreated enzyme was incubated in a total of 13 μL of bu... | US Patent US10829493 (2020) BindingDB Entry DOI: 10.7270/Q2MP56BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha [D842V] (Homo sapiens (Human)) | BDBM472787 (US10829493, Example 13) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Blueprint Medicines Corporation US Patent | Assay Description KIT D816V assay at the APPKM for ATP: In each well of a 384-well assay plate, 0.3 nM of untreated enzyme was incubated in a total of 13 μL of bu... | US Patent US10829493 (2020) BindingDB Entry DOI: 10.7270/Q2MP56BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha [D842V] (Homo sapiens (Human)) | BDBM472788 (US10829493, Example 14) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Blueprint Medicines Corporation US Patent | Assay Description KIT D816V assay at the APPKM for ATP: In each well of a 384-well assay plate, 0.3 nM of untreated enzyme was incubated in a total of 13 μL of bu... | US Patent US10829493 (2020) BindingDB Entry DOI: 10.7270/Q2MP56BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha [D842V] (Homo sapiens (Human)) | BDBM472789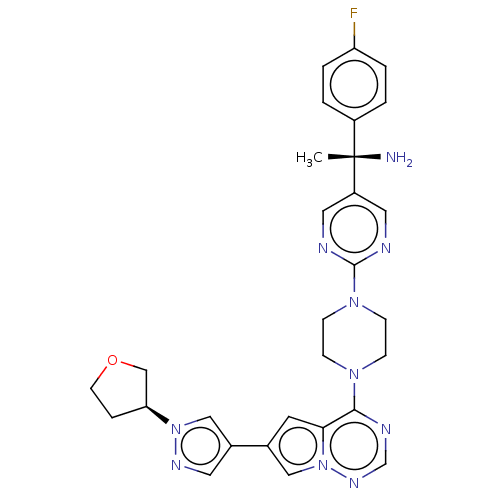 (US10829493, Example 15) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Blueprint Medicines Corporation US Patent | Assay Description KIT D816V assay at the APPKM for ATP: In each well of a 384-well assay plate, 0.3 nM of untreated enzyme was incubated in a total of 13 μL of bu... | US Patent US10829493 (2020) BindingDB Entry DOI: 10.7270/Q2MP56BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha [D842V] (Homo sapiens (Human)) | BDBM472790 (US10829493, Example 16) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Blueprint Medicines Corporation US Patent | Assay Description KIT D816V assay at the APPKM for ATP: In each well of a 384-well assay plate, 0.3 nM of untreated enzyme was incubated in a total of 13 μL of bu... | US Patent US10829493 (2020) BindingDB Entry DOI: 10.7270/Q2MP56BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha [D842V] (Homo sapiens (Human)) | BDBM472791 (US10829493, Example 17) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Blueprint Medicines Corporation US Patent | Assay Description KIT D816V assay at the APPKM for ATP: In each well of a 384-well assay plate, 0.3 nM of untreated enzyme was incubated in a total of 13 μL of bu... | US Patent US10829493 (2020) BindingDB Entry DOI: 10.7270/Q2MP56BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha [D842V] (Homo sapiens (Human)) | BDBM472792 (US10829493, Example 18) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Blueprint Medicines Corporation US Patent | Assay Description KIT D816V assay at the APPKM for ATP: In each well of a 384-well assay plate, 0.3 nM of untreated enzyme was incubated in a total of 13 μL of bu... | US Patent US10829493 (2020) BindingDB Entry DOI: 10.7270/Q2MP56BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha [D842V] (Homo sapiens (Human)) | BDBM472793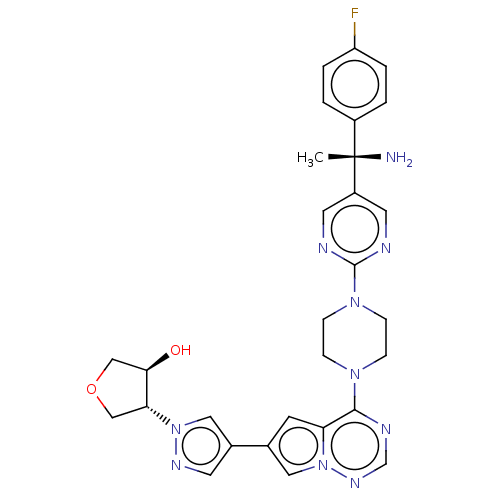 (US10829493, Example 19) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Blueprint Medicines Corporation US Patent | Assay Description KIT D816V assay at the APPKM for ATP: In each well of a 384-well assay plate, 0.3 nM of untreated enzyme was incubated in a total of 13 μL of bu... | US Patent US10829493 (2020) BindingDB Entry DOI: 10.7270/Q2MP56BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha [D842V] (Homo sapiens (Human)) | BDBM472794 (US10829493, Example 20) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Blueprint Medicines Corporation US Patent | Assay Description KIT D816V assay at the APPKM for ATP: In each well of a 384-well assay plate, 0.3 nM of untreated enzyme was incubated in a total of 13 μL of bu... | US Patent US10829493 (2020) BindingDB Entry DOI: 10.7270/Q2MP56BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha [D842V] (Homo sapiens (Human)) | BDBM472795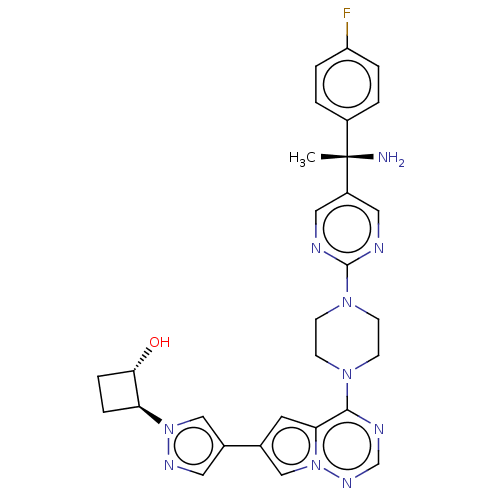 (US10829493, Example 21) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Blueprint Medicines Corporation US Patent | Assay Description KIT D816V assay at the APPKM for ATP: In each well of a 384-well assay plate, 0.3 nM of untreated enzyme was incubated in a total of 13 μL of bu... | US Patent US10829493 (2020) BindingDB Entry DOI: 10.7270/Q2MP56BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha [D842V] (Homo sapiens (Human)) | BDBM472796 (US10829493, Example 22) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Blueprint Medicines Corporation US Patent | Assay Description KIT D816V assay at the APPKM for ATP: In each well of a 384-well assay plate, 0.3 nM of untreated enzyme was incubated in a total of 13 μL of bu... | US Patent US10829493 (2020) BindingDB Entry DOI: 10.7270/Q2MP56BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha [D842V] (Homo sapiens (Human)) | BDBM469288 (US10807985, Compound 63 | US10829493, Example 63 o...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Blueprint Medicines Corporation US Patent | Assay Description KIT D816V assay at the APPKM for ATP: In each well of a 384-well assay plate, 0.3 nM of untreated enzyme was incubated in a total of 13 μL of bu... | US Patent US10829493 (2020) BindingDB Entry DOI: 10.7270/Q2MP56BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50265678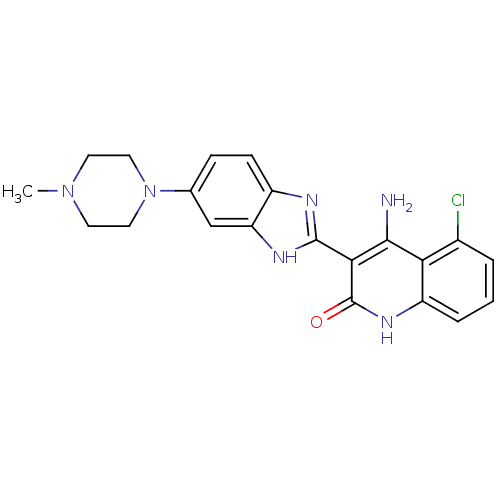 (4-Amino-5-chloro-3-[6-(4-methylpiperazinyl)benzimi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant PDGFRbeta | J Med Chem 52: 278-92 (2009) Article DOI: 10.1021/jm800790t BindingDB Entry DOI: 10.7270/Q2TD9X7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50265773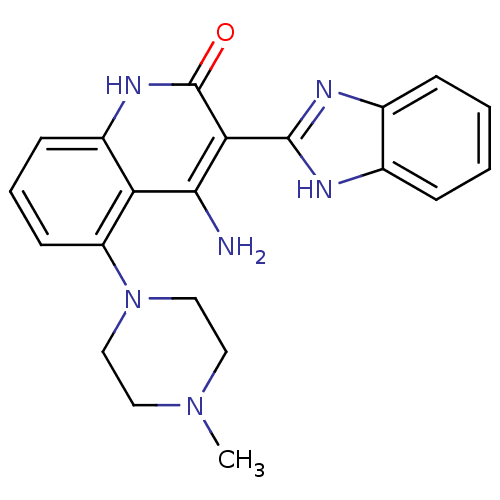 (4-Amino-3-benzimidazol-2-yl-5-(4-methylpiperazinyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant PDGFRbeta | J Med Chem 52: 278-92 (2009) Article DOI: 10.1021/jm800790t BindingDB Entry DOI: 10.7270/Q2TD9X7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50362065 (CHEMBL1940109) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of PDGFRbeta phosphorylation by cell based assay | Bioorg Med Chem Lett 22: 262-6 (2011) Article DOI: 10.1016/j.bmcl.2011.11.019 BindingDB Entry DOI: 10.7270/Q24F1R6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50381931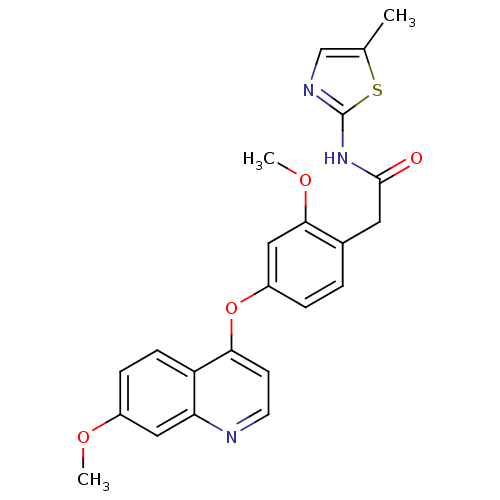 (CHEMBL2023476) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of PDGFRbeta | Bioorg Med Chem Lett 22: 3050-5 (2012) Article DOI: 10.1016/j.bmcl.2012.03.074 BindingDB Entry DOI: 10.7270/Q298881Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha [D842V] (Homo sapiens (Human)) | BDBM472775 (US10829493, Example 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Blueprint Medicines Corporation US Patent | Assay Description KIT D816V assay at the APPKM for ATP: In each well of a 384-well assay plate, 0.3 nM of untreated enzyme was incubated in a total of 13 μL of bu... | US Patent US10829493 (2020) BindingDB Entry DOI: 10.7270/Q2MP56BZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50191974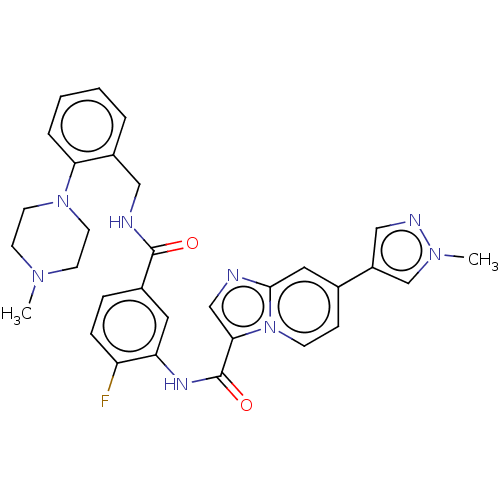 (CHEMBL3913766) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50265750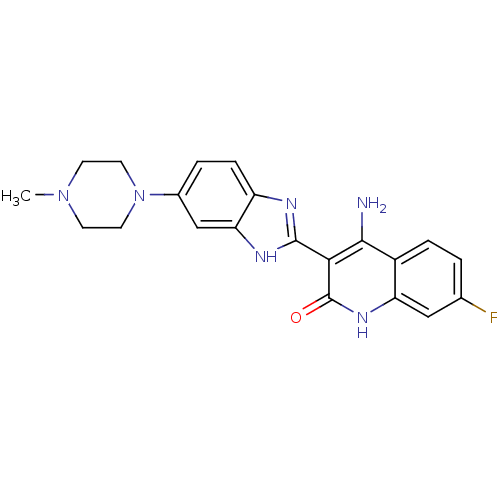 (4-Amino-7-fluoro-3-[6-(4-methylpiperazinyl)benzimi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant PDGFRbeta | J Med Chem 52: 278-92 (2009) Article DOI: 10.1021/jm800790t BindingDB Entry DOI: 10.7270/Q2TD9X7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50117342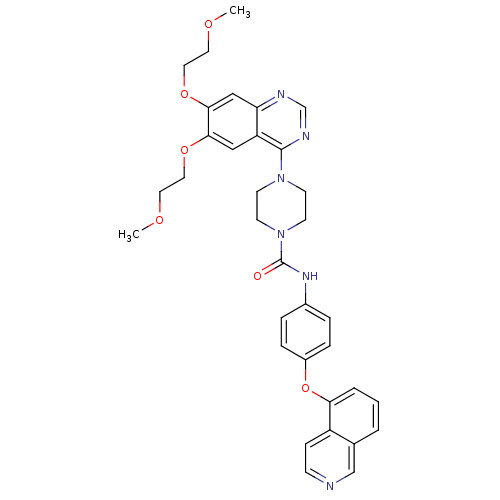 (4-[6,7-Bis-(2-methoxy-ethoxy)-quinazolin-4-yl]-pip...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of platelet-derived growth factor receptor beta phosphorylation in MG63 cells in the absence of plasma | J Med Chem 45: 3772-93 (2002) BindingDB Entry DOI: 10.7270/Q2WW7JD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50117334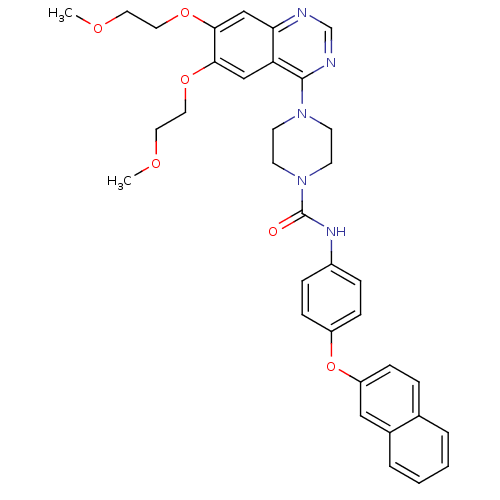 (4-[6,7-Bis-(2-methoxy-ethoxy)-quinazolin-4-yl]-pip...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of platelet-derived growth factor receptor beta phosphorylation in MG63 cells in the absence of plasma | J Med Chem 45: 3772-93 (2002) BindingDB Entry DOI: 10.7270/Q2WW7JD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50381947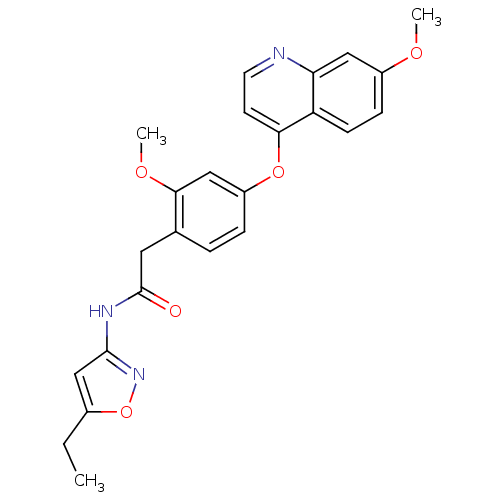 (CHEMBL2023482) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of PDGFRbeta | Bioorg Med Chem Lett 22: 3050-5 (2012) Article DOI: 10.1016/j.bmcl.2012.03.074 BindingDB Entry DOI: 10.7270/Q298881Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50362089 (CHEMBL1940273) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of PDGFRbeta phosphorylation by cell based assay | Bioorg Med Chem Lett 22: 262-6 (2011) Article DOI: 10.1016/j.bmcl.2011.11.019 BindingDB Entry DOI: 10.7270/Q24F1R6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM50431812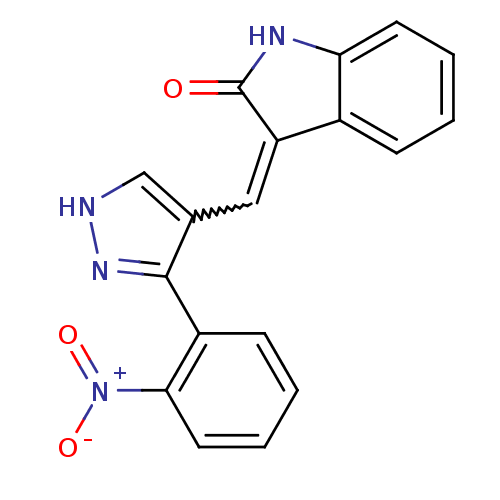 (CHEMBL2347053) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of PDGFRbeta (unknown origin) after 10 mins by mobility shift assay | Bioorg Med Chem 21: 1724-34 (2013) Article DOI: 10.1016/j.bmc.2013.01.047 BindingDB Entry DOI: 10.7270/Q2GQ704N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.418 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human PDGFRalpha using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assay | Citation and Details Article DOI: 10.1016/j.bmc.2018.02.022 BindingDB Entry DOI: 10.7270/Q2DJ5KB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM581370 (US11505527, Compound 4b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description An in vitro kinase assay was performed to evaluate the kinase suppression activity of the most promising cytotoxic candidates 4b, 4j against four dif... | Citation and Details BindingDB Entry DOI: 10.7270/Q25B06BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5043 total ) | Next | Last >> |