 Found 273 hits Enz. Inhib. hit(s) with Target = 'Angiotensin-Converting Enzyme 2'
Found 273 hits Enz. Inhib. hit(s) with Target = 'Angiotensin-Converting Enzyme 2' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21464
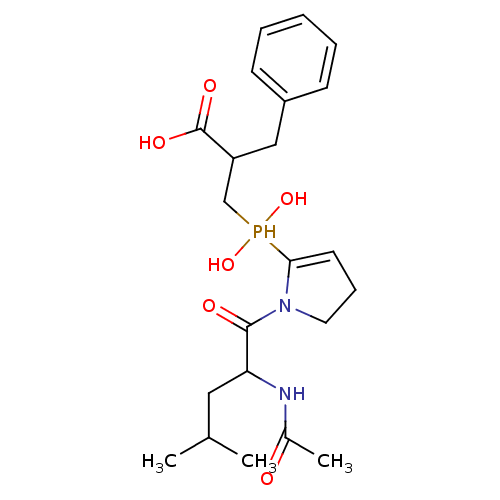
(2-benzyl-3-{[1-(2-acetamido-4-methylpentanoyl)pyrr...)Show SMILES CC(C)CC(NC(C)=O)C(=O)N1CCC=C1P(O)(O)CC(Cc1ccccc1)C(O)=O |c:14| Show InChI InChI=1S/C22H33N2O6P/c1-15(2)12-19(23-16(3)25)21(26)24-11-7-10-20(24)31(29,30)14-18(22(27)28)13-17-8-5-4-6-9-17/h4-6,8-10,15,18-19,29-31H,7,11-14H2,1-3H3,(H,23,25)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | -56.4 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens
| Assay Description
Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... |
J Med Chem 51: 2216-2226 (2008)
Article DOI: 10.1021/jm701275z
BindingDB Entry DOI: 10.7270/Q2QF8R4J |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21474
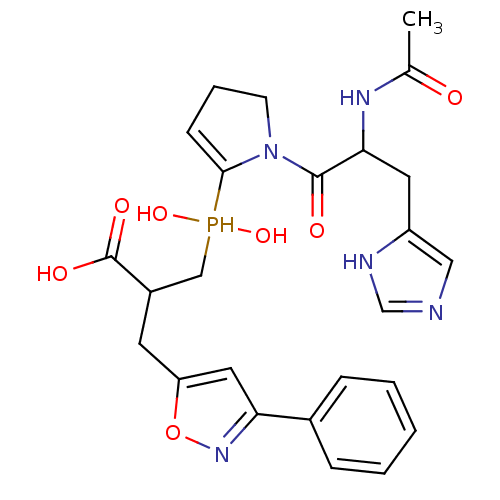
(3-({1-[2-acetamido-3-(1H-imidazol-4-yl)propanoyl]p...)Show SMILES CC(=O)NC(Cc1cnc[nH]1)C(=O)N1CCC=C1P(O)(O)CC(Cc1cc(no1)-c1ccccc1)C(O)=O |c:17| Show InChI InChI=1S/C25H30N5O7P/c1-16(31)28-22(11-19-13-26-15-27-19)24(32)30-9-5-8-23(30)38(35,36)14-18(25(33)34)10-20-12-21(29-37-20)17-6-3-2-4-7-17/h2-4,6-8,12-13,15,18,22,35-36,38H,5,9-11,14H2,1H3,(H,26,27)(H,28,31)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | -53.6 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens
| Assay Description
Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... |
J Med Chem 51: 2216-2226 (2008)
Article DOI: 10.1021/jm701275z
BindingDB Entry DOI: 10.7270/Q2QF8R4J |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Rattus norvegicus) | BDBM50367254

(ENALAPRILAT)Show SMILES C[C@H](N[C@@H](CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r| Show InChI InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12-,14-,15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity towards Angiotensin I converting enzyme of rat brain IgG immobilized enzyme. |
J Med Chem 28: 1208-16 (1985)
BindingDB Entry DOI: 10.7270/Q2VM4CVV |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21470

(2-benzyl-3-({1-[2-acetamido-3-(1H-imidazol-4-yl)pr...)Show SMILES CC(=O)NC(Cc1cnc[nH]1)C(=O)N1CCC=C1P(O)(O)CC(Cc1ccccc1)C(O)=O |c:17| Show InChI InChI=1S/C22H29N4O6P/c1-15(27)25-19(11-18-12-23-14-24-18)21(28)26-9-5-8-20(26)33(31,32)13-17(22(29)30)10-16-6-3-2-4-7-16/h2-4,6-8,12,14,17,19,31-33H,5,9-11,13H2,1H3,(H,23,24)(H,25,27)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | -52.3 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens
| Assay Description
Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... |
J Med Chem 51: 2216-2226 (2008)
Article DOI: 10.1021/jm701275z
BindingDB Entry DOI: 10.7270/Q2QF8R4J |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21473
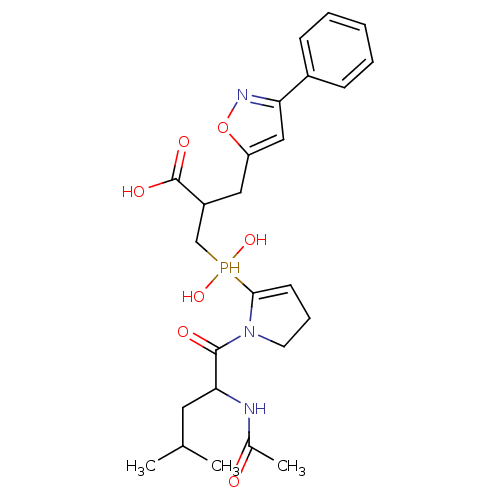
(3-{[1-(2-acetamido-4-methylpentanoyl)pyrrolidin-2-...)Show SMILES CC(C)CC(NC(C)=O)C(=O)N1CCC=C1P(O)(O)CC(Cc1cc(no1)-c1ccccc1)C(O)=O |c:14| Show InChI InChI=1S/C25H34N3O7P/c1-16(2)12-22(26-17(3)29)24(30)28-11-7-10-23(28)36(33,34)15-19(25(31)32)13-20-14-21(27-35-20)18-8-5-4-6-9-18/h4-6,8-10,14,16,19,22,33-34,36H,7,11-13,15H2,1-3H3,(H,26,29)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.25 | -50.8 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens
| Assay Description
Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... |
J Med Chem 51: 2216-2226 (2008)
Article DOI: 10.1021/jm701275z
BindingDB Entry DOI: 10.7270/Q2QF8R4J |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50411731
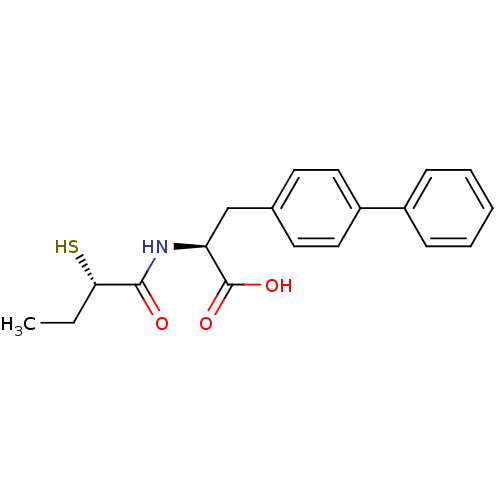
(CHEMBL257726)Show SMILES CC[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C19H21NO3S/c1-2-17(24)18(21)20-16(19(22)23)12-13-8-10-15(11-9-13)14-6-4-3-5-7-14/h3-11,16-17,24H,2,12H2,1H3,(H,20,21)(H,22,23)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50411736
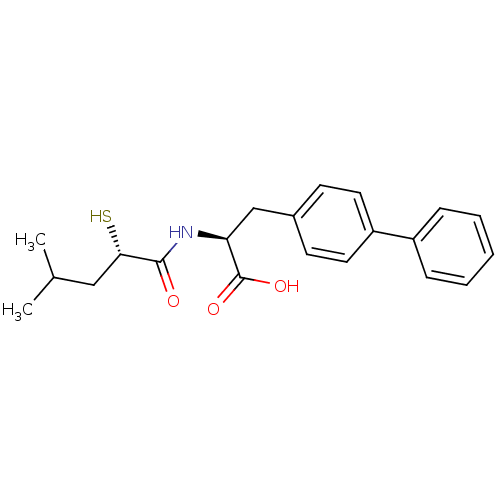
(CHEMBL271225)Show SMILES CC(C)C[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C21H25NO3S/c1-14(2)12-19(26)20(23)22-18(21(24)25)13-15-8-10-17(11-9-15)16-6-4-3-5-7-16/h3-11,14,18-19,26H,12-13H2,1-2H3,(H,22,23)(H,24,25)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50411605
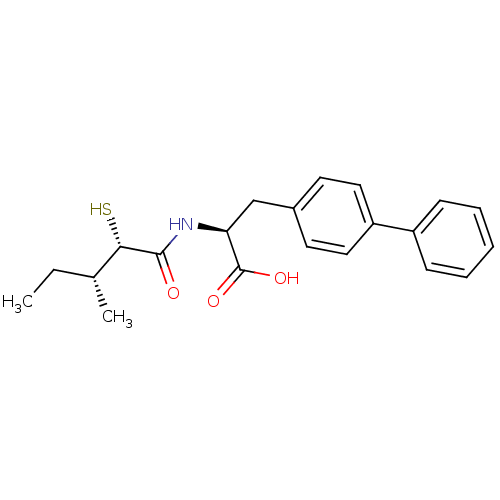
(CHEMBL252391)Show SMILES CC[C@@H](C)[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C21H25NO3S/c1-3-14(2)19(26)20(23)22-18(21(24)25)13-15-9-11-17(12-10-15)16-7-5-4-6-8-16/h4-12,14,18-19,26H,3,13H2,1-2H3,(H,22,23)(H,24,25)/t14-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50411733
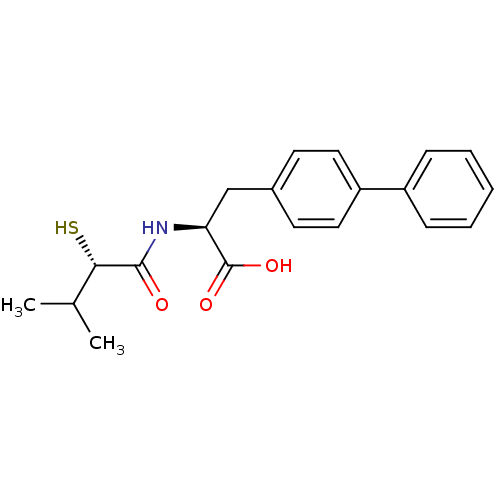
(CHEMBL269997)Show SMILES CC(C)[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C20H23NO3S/c1-13(2)18(25)19(22)21-17(20(23)24)12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,13,17-18,25H,12H2,1-2H3,(H,21,22)(H,23,24)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50411729
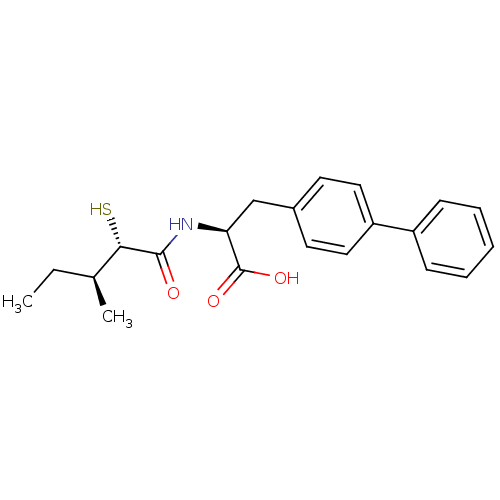
(CHEMBL269996)Show SMILES CC[C@H](C)[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C21H25NO3S/c1-3-14(2)19(26)20(23)22-18(21(24)25)13-15-9-11-17(12-10-15)16-7-5-4-6-8-16/h4-12,14,18-19,26H,3,13H2,1-2H3,(H,22,23)(H,24,25)/t14-,18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50411730
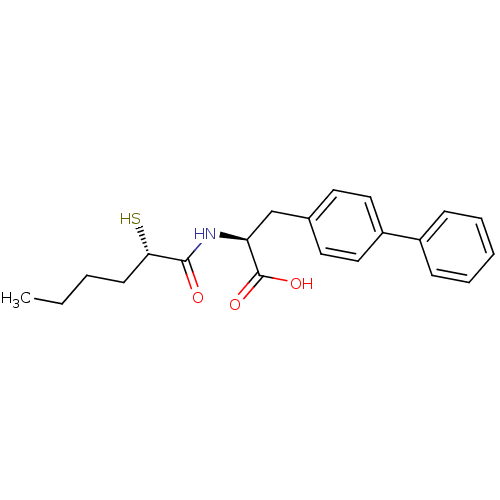
(CHEMBL257270)Show SMILES CCCC[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C21H25NO3S/c1-2-3-9-19(26)20(23)22-18(21(24)25)14-15-10-12-17(13-11-15)16-7-5-4-6-8-16/h4-8,10-13,18-19,26H,2-3,9,14H2,1H3,(H,22,23)(H,24,25)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50411737
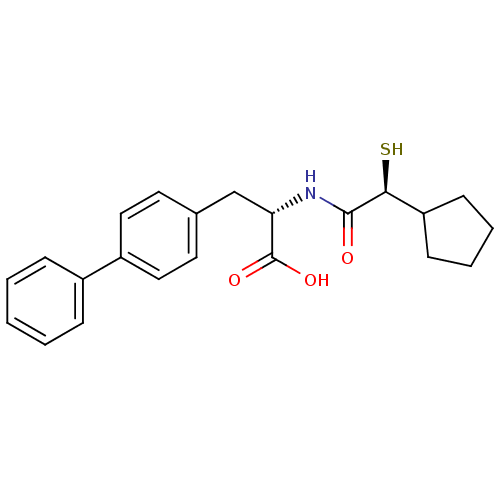
(CHEMBL404117)Show SMILES OC(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@@H](S)C1CCCC1 Show InChI InChI=1S/C22H25NO3S/c24-21(20(27)18-8-4-5-9-18)23-19(22(25)26)14-15-10-12-17(13-11-15)16-6-2-1-3-7-16/h1-3,6-7,10-13,18-20,27H,4-5,8-9,14H2,(H,23,24)(H,25,26)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50411728
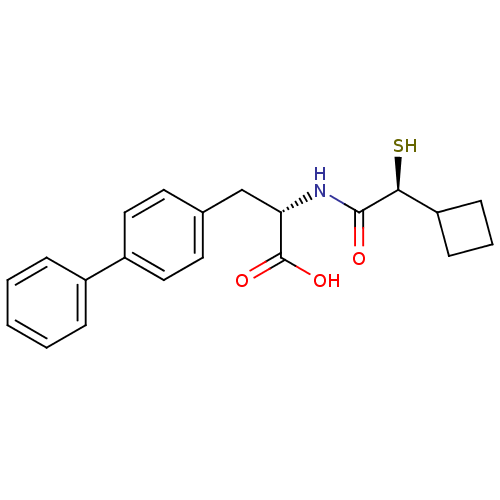
(CHEMBL257229)Show SMILES OC(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@@H](S)C1CCC1 Show InChI InChI=1S/C21H23NO3S/c23-20(19(26)17-7-4-8-17)22-18(21(24)25)13-14-9-11-16(12-10-14)15-5-2-1-3-6-15/h1-3,5-6,9-12,17-19,26H,4,7-8,13H2,(H,22,23)(H,24,25)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | CHEMBL5275219
Show InChI InChI=1S/C16H22N2OS/c1-2-3-4-5-9-12-18-15(19)14(17-16(18)20)13-10-7-6-8-11-13/h6-8,10-11,19H,2-5,9,12H2,1H3,(H,17,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
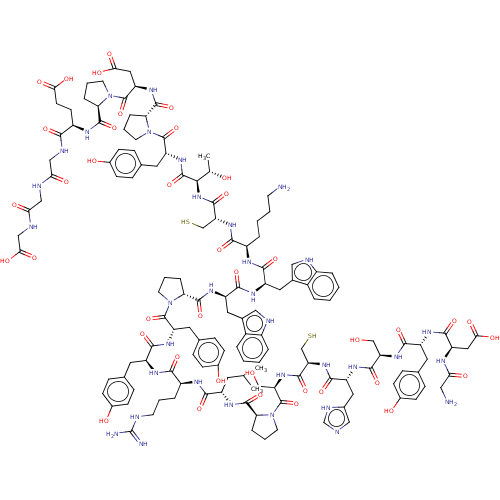
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50326045
(CHEMBL1240682)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](CC(O)=O)NC(=O)CN)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CS)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N1CCC[C@H]1C(O)=O |r,wU:106.112,55.62,21.24,147.156,91.94,179.190,4.4,124.131,wD:153.162,175.187,190.203,8.7,43.54,37.41,27.35,15.18,138.147,154.165,160.169,110.115,68.70,79.81,(26.05,-7.23,;26.13,-5.69,;24.83,-4.85,;27.49,-4.99,;27.57,-3.46,;28.94,-2.76,;30.27,-3.54,;30.25,-5.08,;31.52,-2.65,;31.53,-1.11,;33,-.64,;33.89,-1.89,;32.98,-3.13,;33.67,-4.51,;32.88,-5.83,;35.21,-4.52,;35.99,-3.2,;37.53,-3.21,;35.96,-5.86,;37.5,-5.87,;38.28,-4.55,;38.26,-7.21,;37.48,-8.54,;38.23,-9.88,;39.8,-7.22,;40.58,-5.9,;39.82,-4.56,;42.12,-5.92,;42.78,-4.52,;41.91,-3.26,;40.37,-3.21,;39.94,-1.74,;41.21,-.87,;42.43,-1.81,;42.88,-7.25,;44.42,-7.27,;45.19,-5.94,;45.17,-8.61,;46.71,-8.62,;47.5,-7.31,;44.39,-9.93,;45.15,-11.27,;44.37,-12.6,;46.69,-11.28,;47.47,-9.96,;49.01,-9.98,;49.79,-8.65,;51.33,-8.66,;52.09,-10,;53.64,-10.02,;51.3,-11.33,;49.77,-11.31,;47.44,-12.63,;46.66,-13.96,;45.12,-13.94,;47.42,-15.29,;48.96,-15.31,;49.74,-13.98,;51.29,-14,;48.98,-12.64,;46.64,-16.62,;45.1,-16.6,;44.32,-17.93,;44.35,-15.27,;42.81,-15.25,;26.27,-2.62,;26.36,-1.08,;24.9,-3.31,;23.6,-2.48,;23.57,-.95,;22.22,-.2,;22.2,1.35,;20.86,2.11,;20.82,3.65,;22.14,4.44,;19.48,4.41,;22.23,-3.18,;22.15,-4.72,;20.94,-2.34,;19.56,-3.04,;18.27,-2.2,;16.9,-2.9,;15.61,-2.07,;14.23,-2.77,;14.16,-4.3,;12.81,-4.98,;15.45,-5.14,;16.82,-4.45,;19.49,-4.58,;20.78,-5.41,;18.11,-5.27,;18.02,-6.8,;19.74,-7.82,;19.73,-9.36,;21.05,-10.15,;21.03,-11.69,;19.69,-12.44,;19.69,-13.96,;18.36,-11.66,;18.39,-10.12,;16.24,-7.72,;14.96,-6.88,;16.08,-9.25,;17.22,-10.47,;16.41,-11.95,;14.76,-11.64,;14.83,-10.1,;13.48,-9.35,;13.46,-7.8,;12.16,-10.14,;10.81,-9.4,;10.78,-7.87,;9.43,-7.12,;8.19,-8.02,;6.94,-7.12,;7.43,-5.66,;6.65,-4.32,;7.43,-2.99,;8.96,-2.98,;9.73,-4.32,;8.97,-5.66,;9.49,-10.2,;9.52,-11.73,;8.14,-9.44,;6.82,-10.23,;6.85,-11.77,;5.53,-12.56,;4.26,-11.68,;3.04,-12.62,;3.55,-14.07,;2.81,-15.42,;3.61,-16.73,;5.14,-16.7,;5.88,-15.35,;5.08,-14.04,;5.48,-9.48,;5.45,-7.94,;4.15,-10.26,;2.78,-9.54,;2.77,-7.99,;1.41,-7.26,;1.38,-5.71,;.03,-4.97,;.03,-3.4,;1.47,-10.32,;1.49,-11.86,;.13,-9.56,;-1.18,-10.37,;-1.15,-11.9,;-2.49,-12.7,;-2.54,-9.62,;-2.56,-8.08,;-3.85,-10.41,;-5.19,-9.66,;-5.21,-8.12,;-3.91,-7.33,;-6.56,-7.36,;-6.52,-10.46,;-6.49,-12,;-7.86,-9.7,;-9.19,-10.48,;-10.54,-9.74,;-10.56,-8.2,;-9.24,-7.41,;-9.27,-5.88,;-10.64,-5.13,;-10.63,-3.57,;-11.94,-5.92,;-11.91,-7.45,;-9.16,-12.03,;-7.82,-12.77,;-10.34,-13.01,;-11.99,-12.72,;-12.8,-14.2,;-11.64,-15.41,;-10.36,-14.51,;-9.08,-15.37,;-7.74,-14.61,;-9.07,-16.91,;-7.76,-17.71,;-6.42,-16.97,;-5.09,-17.75,;-3.72,-16.98,;-5.1,-19.29,;-7.78,-19.26,;-6.45,-20.05,;-8.97,-20.22,;-10.61,-19.91,;-11.45,-21.38,;-10.29,-22.6,;-9,-21.72,;-7.67,-22.47,;-6.33,-21.64,;-7.62,-24,)| Show InChI InChI=1S/C128H168N30O34S2/c1-66(2)47-85(145-119(183)100-22-13-45-157(100)126(190)96(62-160)151-117(181)97(63-193)153-114(178)90(54-74-60-133-65-137-74)144-116(180)95(61-159)150-111(175)87(49-69-27-35-76(163)36-28-69)142-115(179)91(55-104(167)168)138-103(166)57-130)109(173)140-84(20-10-42-134-128(131)132)107(171)141-86(48-68-25-33-75(162)34-26-68)110(174)147-92(50-70-29-37-77(164)38-30-70)123(187)155-43-11-21-99(155)120(184)146-89(53-73-59-136-82-18-7-5-16-80(73)82)113(177)143-88(52-72-58-135-81-17-6-4-15-79(72)81)112(176)139-83(19-8-9-41-129)108(172)152-98(64-194)118(182)154-106(67(3)161)122(186)149-93(51-71-31-39-78(165)40-32-71)124(188)156-44-12-23-101(156)121(185)148-94(56-105(169)170)125(189)158-46-14-24-102(158)127(191)192/h4-7,15-18,25-40,58-60,65-67,83-102,106,135-136,159-165,193-194H,8-14,19-24,41-57,61-64,129-130H2,1-3H3,(H,133,137)(H,138,166)(H,139,176)(H,140,173)(H,141,171)(H,142,179)(H,143,177)(H,144,180)(H,145,183)(H,146,184)(H,147,174)(H,148,185)(H,149,186)(H,150,175)(H,151,181)(H,152,172)(H,153,178)(H,154,182)(H,167,168)(H,169,170)(H,191,192)(H4,131,132,134)/t67-,83+,84+,85+,86+,87+,88+,89+,90+,91-,92+,93+,94+,95+,96+,97+,98+,99+,100+,101+,102+,106+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Molecular Biology, Medical Research Council
Curated by ChEMBL
| Assay Description
Binding affinity to angiotensin converting enzyme 2 |
Nat Chem Biol 5: 502-7 (2009)
Article DOI: 10.1038/nchembio.184
BindingDB Entry DOI: 10.7270/Q2Z60P9P |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(BOVINE) | BDBM85558
(Ang IV Nor1)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCCN)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C39H52N8O8/c1-3-24(2)34(46-35(50)29(43-33(49)12-7-17-40)19-26-13-15-28(48)16-14-26)37(52)44-30(21-27-22-41-23-42-27)38(53)47-18-8-11-32(47)36(51)45-31(39(54)55)20-25-9-5-4-6-10-25/h4-6,9-10,13-16,22-24,29-32,34,48H,3,7-8,11-12,17-21,40H2,1-2H3,(H,41,42)(H,43,49)(H,44,52)(H,45,51)(H,46,50)(H,54,55)/t24-,29-,30-,31-,32-,34-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 3.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 291: 1242-9 (1999)
BindingDB Entry DOI: 10.7270/Q2377783 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(BOVINE) | BDBM85550

(Ang IV | CAS_12676-15-2 | CHEMBL261120 | cid_12381...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C40H54N8O8/c1-5-24(4)34(47-35(50)29(44-37(52)33(41)23(2)3)18-26-13-15-28(49)16-14-26)38(53)45-30(20-27-21-42-22-43-27)39(54)48-17-9-12-32(48)36(51)46-31(40(55)56)19-25-10-7-6-8-11-25/h6-8,10-11,13-16,21-24,29-34,49H,5,9,12,17-20,41H2,1-4H3,(H,42,43)(H,44,52)(H,45,53)(H,46,51)(H,47,50)(H,55,56)/t24-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 291: 1242-9 (1999)
BindingDB Entry DOI: 10.7270/Q2377783 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(BOVINE) | BDBM85550

(Ang IV | CAS_12676-15-2 | CHEMBL261120 | cid_12381...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C40H54N8O8/c1-5-24(4)34(47-35(50)29(44-37(52)33(41)23(2)3)18-26-13-15-28(49)16-14-26)38(53)45-30(20-27-21-42-22-43-27)39(54)48-17-9-12-32(48)36(51)46-31(40(55)56)19-25-10-7-6-8-11-25/h6-8,10-11,13-16,21-24,29-34,49H,5,9,12,17-20,41H2,1-4H3,(H,42,43)(H,44,52)(H,45,53)(H,46,51)(H,47,50)(H,55,56)/t24-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 291: 1242-9 (1999)
BindingDB Entry DOI: 10.7270/Q2377783 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(BOVINE) | BDBM85542

(Divalinal Ang IV)Show SMILES CC(C)[C@H](N)CN[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H](CN[C@@H](Cc1cnc[nH]1)C(=O)CN1CCCC1C(=O)NC(C(O)=O)c1ccccc1)C(C)C |r| Show InChI InChI=1S/C39H56N8O6/c1-24(2)30(40)20-42-32(17-26-12-14-29(48)15-13-26)37(50)45-33(25(3)4)21-43-31(18-28-19-41-23-44-28)35(49)22-47-16-8-11-34(47)38(51)46-36(39(52)53)27-9-6-5-7-10-27/h5-7,9-10,12-15,19,23-25,30-34,36,42-43,48H,8,11,16-18,20-22,40H2,1-4H3,(H,41,44)(H,45,50)(H,46,51)(H,52,53)/t30-,31+,32+,33-,34?,36?/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 4.07 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 291: 1242-9 (1999)
BindingDB Entry DOI: 10.7270/Q2377783 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21468
(2-benzyl-3-{[1-(2-acetamido-3-phenylpropanoyl)pyrr...)Show SMILES CC(=O)NC(Cc1ccccc1)C(=O)N1CCC=C1P(O)(O)CC(Cc1ccccc1)C(O)=O |c:18| Show InChI InChI=1S/C25H31N2O6P/c1-18(28)26-22(16-20-11-6-3-7-12-20)24(29)27-14-8-13-23(27)34(32,33)17-21(25(30)31)15-19-9-4-2-5-10-19/h2-7,9-13,21-22,32-34H,8,14-17H2,1H3,(H,26,28)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20 | -47.3 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens
| Assay Description
Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... |
J Med Chem 51: 2216-2226 (2008)
Article DOI: 10.1021/jm701275z
BindingDB Entry DOI: 10.7270/Q2QF8R4J |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21467
(2-benzyl-3-({1-[2-acetamido-3-(4-hydroxyphenyl)pro...)Show SMILES CC(=O)NC(Cc1ccc(O)cc1)C(=O)N1CCC=C1P(O)(O)CC(Cc1ccccc1)C(O)=O |c:19| Show InChI InChI=1S/C25H31N2O7P/c1-17(28)26-22(15-19-9-11-21(29)12-10-19)24(30)27-13-5-8-23(27)35(33,34)16-20(25(31)32)14-18-6-3-2-4-7-18/h2-4,6-12,20,22,29,33-35H,5,13-16H2,1H3,(H,26,28)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20 | -47.3 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens
| Assay Description
Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... |
J Med Chem 51: 2216-2226 (2008)
Article DOI: 10.1021/jm701275z
BindingDB Entry DOI: 10.7270/Q2QF8R4J |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(BOVINE) | BDBM85550

(Ang IV | CAS_12676-15-2 | CHEMBL261120 | cid_12381...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C40H54N8O8/c1-5-24(4)34(47-35(50)29(44-37(52)33(41)23(2)3)18-26-13-15-28(49)16-14-26)38(53)45-30(20-27-21-42-22-43-27)39(54)48-17-9-12-32(48)36(51)46-31(40(55)56)19-25-10-7-6-8-11-25/h6-8,10-11,13-16,21-24,29-34,49H,5,9,12,17-20,41H2,1-4H3,(H,42,43)(H,44,52)(H,45,53)(H,46,51)(H,47,50)(H,55,56)/t24-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 291: 1242-9 (1999)
BindingDB Entry DOI: 10.7270/Q2377783 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(BOVINE) | BDBM85550

(Ang IV | CAS_12676-15-2 | CHEMBL261120 | cid_12381...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C40H54N8O8/c1-5-24(4)34(47-35(50)29(44-37(52)33(41)23(2)3)18-26-13-15-28(49)16-14-26)38(53)45-30(20-27-21-42-22-43-27)39(54)48-17-9-12-32(48)36(51)46-31(40(55)56)19-25-10-7-6-8-11-25/h6-8,10-11,13-16,21-24,29-34,49H,5,9,12,17-20,41H2,1-4H3,(H,42,43)(H,44,52)(H,45,53)(H,46,51)(H,47,50)(H,55,56)/t24-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 291: 1242-9 (1999)
BindingDB Entry DOI: 10.7270/Q2377783 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(BOVINE) | BDBM85542

(Divalinal Ang IV)Show SMILES CC(C)[C@H](N)CN[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H](CN[C@@H](Cc1cnc[nH]1)C(=O)CN1CCCC1C(=O)NC(C(O)=O)c1ccccc1)C(C)C |r| Show InChI InChI=1S/C39H56N8O6/c1-24(2)30(40)20-42-32(17-26-12-14-29(48)15-13-26)37(50)45-33(25(3)4)21-43-31(18-28-19-41-23-44-28)35(49)22-47-16-8-11-34(47)38(51)46-36(39(52)53)27-9-6-5-7-10-27/h5-7,9-10,12-15,19,23-25,30-34,36,42-43,48H,8,11,16-18,20-22,40H2,1-4H3,(H,41,44)(H,45,50)(H,46,51)(H,52,53)/t30-,31+,32+,33-,34?,36?/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 6.39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 291: 1242-9 (1999)
BindingDB Entry DOI: 10.7270/Q2377783 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21465

(3-{[1-(6-amino-2-acetamidohexanoyl)pyrrolidin-2-yl...)Show SMILES CC(=O)NC(CCCCN)C(=O)N1CCC=C1P(O)(O)CC(Cc1ccccc1)C(O)=O |c:15| Show InChI InChI=1S/C22H34N3O6P/c1-16(26)24-19(10-5-6-12-23)21(27)25-13-7-11-20(25)32(30,31)15-18(22(28)29)14-17-8-3-2-4-9-17/h2-4,8-9,11,18-19,30-32H,5-7,10,12-15,23H2,1H3,(H,24,26)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.5 | -46.7 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens
| Assay Description
Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... |
J Med Chem 51: 2216-2226 (2008)
Article DOI: 10.1021/jm701275z
BindingDB Entry DOI: 10.7270/Q2QF8R4J |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21469
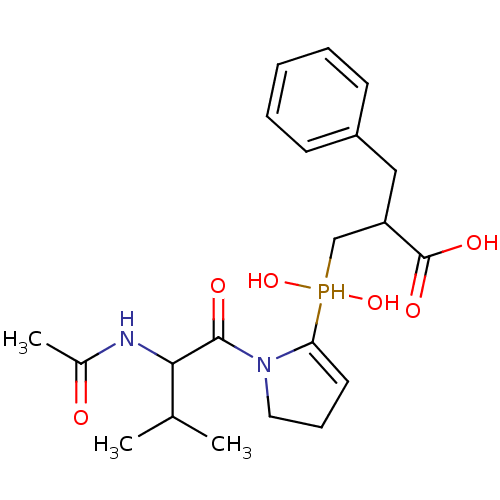
(2-benzyl-3-{[1-(2-acetamido-3-methylbutanoyl)pyrro...)Show SMILES CC(C)C(NC(C)=O)C(=O)N1CCC=C1P(O)(O)CC(Cc1ccccc1)C(O)=O |c:13| Show InChI InChI=1S/C21H31N2O6P/c1-14(2)19(22-15(3)24)20(25)23-11-7-10-18(23)30(28,29)13-17(21(26)27)12-16-8-5-4-6-9-16/h4-6,8-10,14,17,19,28-30H,7,11-13H2,1-3H3,(H,22,24)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.60 | -46.7 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens
| Assay Description
Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... |
J Med Chem 51: 2216-2226 (2008)
Article DOI: 10.1021/jm701275z
BindingDB Entry DOI: 10.7270/Q2QF8R4J |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50411734

(CHEMBL257727)Show SMILES C[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C18H19NO3S/c1-12(23)17(20)19-16(18(21)22)11-13-7-9-15(10-8-13)14-5-3-2-4-6-14/h2-10,12,16,23H,11H2,1H3,(H,19,20)(H,21,22)/t12-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21466
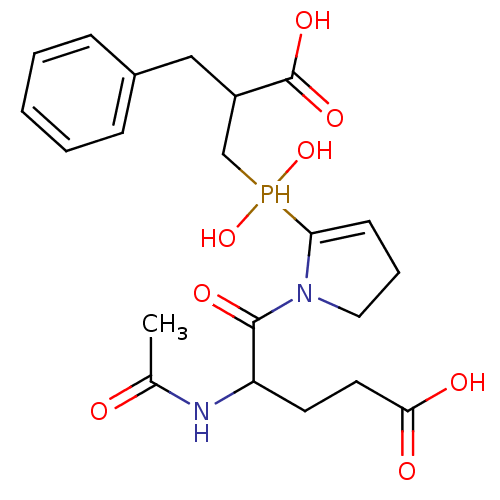
(5-{2-[(2-benzyl-2-carboxyethyl)(hydroxy)phosphoryl...)Show SMILES CC(=O)NC(CCC(O)=O)C(=O)N1CCC=C1P(O)(O)CC(Cc1ccccc1)C(O)=O |c:15| Show InChI InChI=1S/C21H29N2O8P/c1-14(24)22-17(9-10-19(25)26)20(27)23-11-5-8-18(23)32(30,31)13-16(21(28)29)12-15-6-3-2-4-7-15/h2-4,6-8,16-17,30-32H,5,9-13H2,1H3,(H,22,24)(H,25,26)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | -46.5 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens
| Assay Description
Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... |
J Med Chem 51: 2216-2226 (2008)
Article DOI: 10.1021/jm701275z
BindingDB Entry DOI: 10.7270/Q2QF8R4J |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50411725
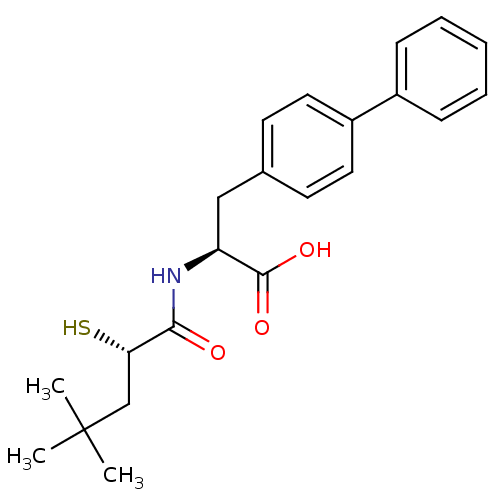
(CHEMBL271223)Show SMILES CC(C)(C)C[C@H](S)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C22H27NO3S/c1-22(2,3)14-19(27)20(24)23-18(21(25)26)13-15-9-11-17(12-10-15)16-7-5-4-6-8-16/h4-12,18-19,27H,13-14H2,1-3H3,(H,23,24)(H,25,26)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(BOVINE) | BDBM85546

(Ang(3-9))Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](C(O)=O)c1cnc[nH]1 |r| Show InChI InChI=1S/C45H59N11O9/c1-5-26(4)37(54-39(58)32(52-42(61)36(46)25(2)3)19-28-13-15-30(57)16-14-28)43(62)53-33(20-29-21-47-23-49-29)44(63)56-17-9-12-35(56)41(60)51-31(18-27-10-7-6-8-11-27)40(59)55-38(45(64)65)34-22-48-24-50-34/h6-8,10-11,13-16,21-26,31-33,35-38,57H,5,9,12,17-20,46H2,1-4H3,(H,47,49)(H,48,50)(H,51,60)(H,52,61)(H,53,62)(H,54,58)(H,55,59)(H,64,65)/t26-,31-,32-,33-,35-,36-,37-,38+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 7.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 291: 1242-9 (1999)
BindingDB Entry DOI: 10.7270/Q2377783 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21463
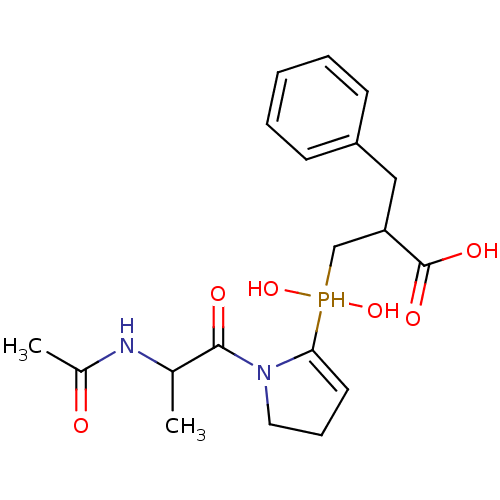
(2-benzyl-3-{[1-(2-acetamidopropanoyl)pyrrolidin-2-...)Show SMILES CC(NC(C)=O)C(=O)N1CCC=C1P(O)(O)CC(Cc1ccccc1)C(O)=O |c:11| Show InChI InChI=1S/C19H27N2O6P/c1-13(20-14(2)22)18(23)21-10-6-9-17(21)28(26,27)12-16(19(24)25)11-15-7-4-3-5-8-15/h3-5,7-9,13,16,26-28H,6,10-12H2,1-2H3,(H,20,22)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.5 | -46.4 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens
| Assay Description
Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... |
J Med Chem 51: 2216-2226 (2008)
Article DOI: 10.1021/jm701275z
BindingDB Entry DOI: 10.7270/Q2QF8R4J |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(BOVINE) | BDBM85558

(Ang IV Nor1)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)CCCN)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C39H52N8O8/c1-3-24(2)34(46-35(50)29(43-33(49)12-7-17-40)19-26-13-15-28(48)16-14-26)37(52)44-30(21-27-22-41-23-42-27)38(53)47-18-8-11-32(47)36(51)45-31(39(54)55)20-25-9-5-4-6-10-25/h4-6,9-10,13-16,22-24,29-32,34,48H,3,7-8,11-12,17-21,40H2,1-2H3,(H,41,42)(H,43,49)(H,44,52)(H,45,51)(H,46,50)(H,54,55)/t24-,29-,30-,31-,32-,34-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 8.49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 291: 1242-9 (1999)
BindingDB Entry DOI: 10.7270/Q2377783 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(BOVINE) | BDBM85545
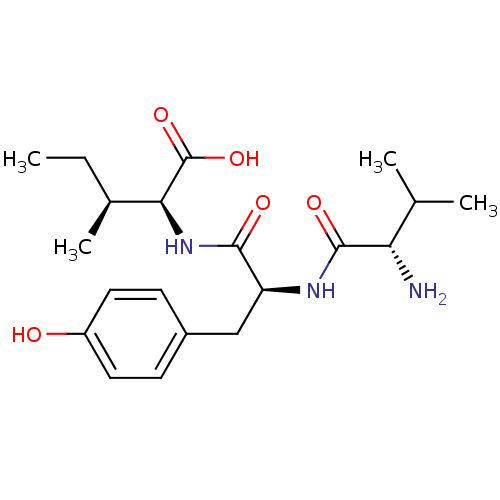
(Ang(3-5))Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)C(C)C)C(O)=O Show InChI InChI=1S/C20H31N3O5/c1-5-12(4)17(20(27)28)23-18(25)15(22-19(26)16(21)11(2)3)10-13-6-8-14(24)9-7-13/h6-9,11-12,15-17,24H,5,10,21H2,1-4H3,(H,22,26)(H,23,25)(H,27,28)/t12-,15-,16-,17-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 291: 1242-9 (1999)
BindingDB Entry DOI: 10.7270/Q2377783 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(BOVINE) | BDBM85553
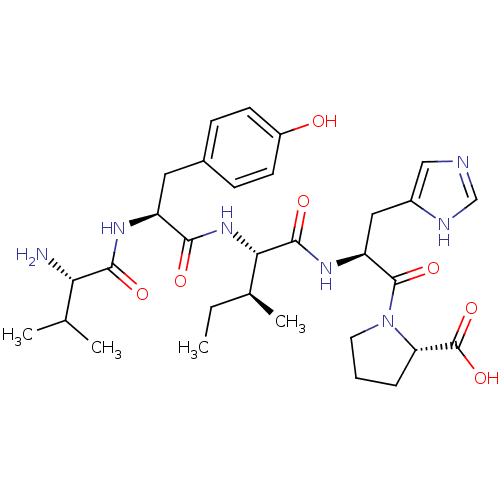
(Ang(3-7))Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C31H45N7O7/c1-5-18(4)26(37-27(40)22(35-28(41)25(32)17(2)3)13-19-8-10-21(39)11-9-19)29(42)36-23(14-20-15-33-16-34-20)30(43)38-12-6-7-24(38)31(44)45/h8-11,15-18,22-26,39H,5-7,12-14,32H2,1-4H3,(H,33,34)(H,35,41)(H,36,42)(H,37,40)(H,44,45)/t18-,22-,23-,24-,25-,26-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 10.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 291: 1242-9 (1999)
BindingDB Entry DOI: 10.7270/Q2377783 |
More data for this
Ligand-Target Pair | |
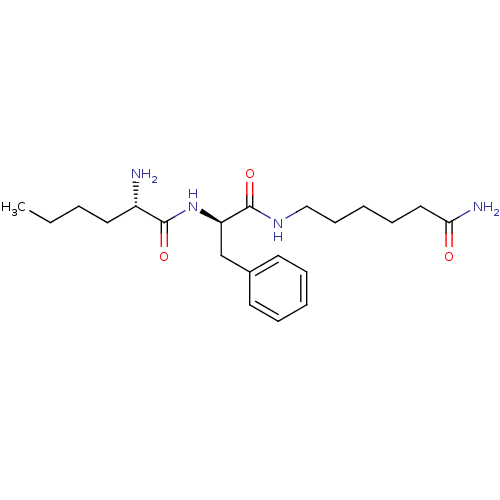
Angiotensin-converting enzyme 2
(BOVINE) | BDBM85549
(NleYI-6-hexamide)Show SMILES CCCC[C@H](N)C(=O)N[C@H](Cc1ccccc1)C(=O)NCCCCCC(N)=O |r| Show InChI InChI=1S/C21H34N4O3/c1-2-3-12-17(22)20(27)25-18(15-16-10-6-4-7-11-16)21(28)24-14-9-5-8-13-19(23)26/h4,6-7,10-11,17-18H,2-3,5,8-9,12-15,22H2,1H3,(H2,23,26)(H,24,28)(H,25,27)/t17-,18+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 13.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 291: 1242-9 (1999)
BindingDB Entry DOI: 10.7270/Q2377783 |
More data for this
Ligand-Target Pair | |
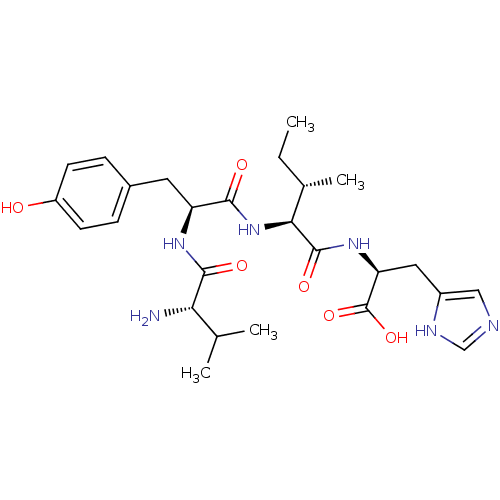
Angiotensin-converting enzyme 2
(BOVINE) | BDBM85547
(Ang(3-6))Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(O)=O Show InChI InChI=1S/C26H38N6O6/c1-5-15(4)22(25(36)31-20(26(37)38)11-17-12-28-13-29-17)32-23(34)19(30-24(35)21(27)14(2)3)10-16-6-8-18(33)9-7-16/h6-9,12-15,19-22,33H,5,10-11,27H2,1-4H3,(H,28,29)(H,30,35)(H,31,36)(H,32,34)(H,37,38)/t15-,19-,20-,21-,22-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 14.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 291: 1242-9 (1999)
BindingDB Entry DOI: 10.7270/Q2377783 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(BOVINE) | BDBM85548
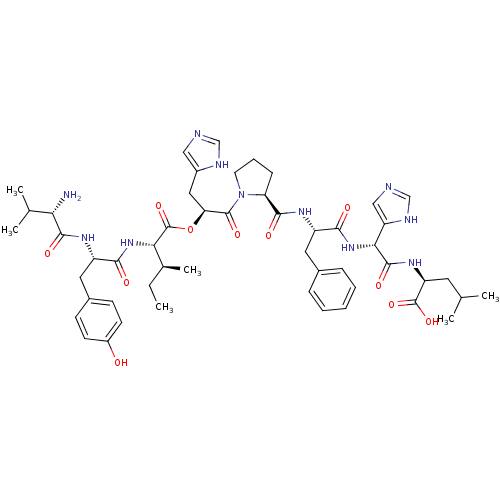
(Ang(3-10))Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)C(C)C)C(=O)O[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](C(=O)N[C@@H](CC(C)C)C(O)=O)c1cnc[nH]1 |r| Show InChI InChI=1S/C51H69N11O11/c1-7-30(6)42(60-44(64)36(58-47(67)41(52)29(4)5)22-32-15-17-34(63)18-16-32)51(72)73-40(23-33-24-53-26-55-33)49(69)62-19-11-14-39(62)46(66)57-35(21-31-12-9-8-10-13-31)45(65)61-43(38-25-54-27-56-38)48(68)59-37(50(70)71)20-28(2)3/h8-10,12-13,15-18,24-30,35-37,39-43,63H,7,11,14,19-23,52H2,1-6H3,(H,53,55)(H,54,56)(H,57,66)(H,58,67)(H,59,68)(H,60,64)(H,61,65)(H,70,71)/t30-,35-,36-,37-,39-,40-,41-,42-,43+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 18.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 291: 1242-9 (1999)
BindingDB Entry DOI: 10.7270/Q2377783 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(BOVINE) | BDBM85543
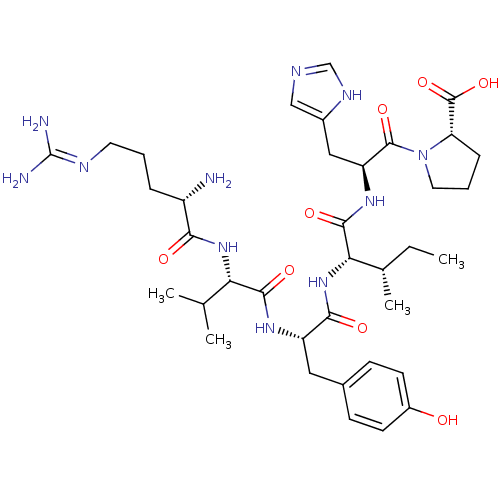
(Ang(2-7))Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@@H](N)CCCN=C(N)N)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(O)=O |wU:39.39,8.17,20.21,wD:4.4,24.25,52.55,2.2,(13.52,3.66,;12.19,2.89,;12.19,1.35,;13.52,.58,;10.85,.58,;10.85,-.96,;12.19,-1.73,;13.52,-.96,;12.19,-3.27,;10.85,-4.04,;10.85,-5.58,;9.52,-6.35,;9.52,-7.89,;10.85,-8.66,;10.85,-10.2,;12.19,-7.89,;12.19,-6.35,;13.52,-4.04,;14.85,-3.27,;14.85,-1.73,;16.19,-4.04,;17.52,-3.27,;18.85,-4.04,;18.85,-5.58,;20.19,-3.27,;21.52,-4.04,;20.19,-1.73,;21.52,-.96,;21.52,.58,;22.86,1.35,;22.86,2.89,;24.19,3.66,;21.52,3.66,;16.19,-5.58,;17.52,-6.35,;14.85,-6.35,;9.52,1.35,;8.18,.58,;9.52,2.89,;8.18,3.66,;6.85,2.89,;5.52,3.66,;5.36,5.19,;3.85,5.51,;3.08,4.18,;4.11,3.03,;8.18,5.2,;6.85,5.97,;9.52,5.97,;10.93,5.34,;11.96,6.49,;11.19,7.82,;9.68,7.5,;8.53,8.53,;8.86,10.04,;7.07,8.06,)| Show InChI InChI=1S/C37H57N11O8/c1-5-21(4)30(34(53)45-27(17-23-18-41-19-43-23)35(54)48-15-7-9-28(48)36(55)56)47-32(51)26(16-22-10-12-24(49)13-11-22)44-33(52)29(20(2)3)46-31(50)25(38)8-6-14-42-37(39)40/h10-13,18-21,25-30,49H,5-9,14-17,38H2,1-4H3,(H,41,43)(H,44,52)(H,45,53)(H,46,50)(H,47,51)(H,55,56)(H4,39,40,42)/t21-,25-,26-,27-,28-,29-,30-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 33.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 291: 1242-9 (1999)
BindingDB Entry DOI: 10.7270/Q2377783 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(BOVINE) | BDBM85557
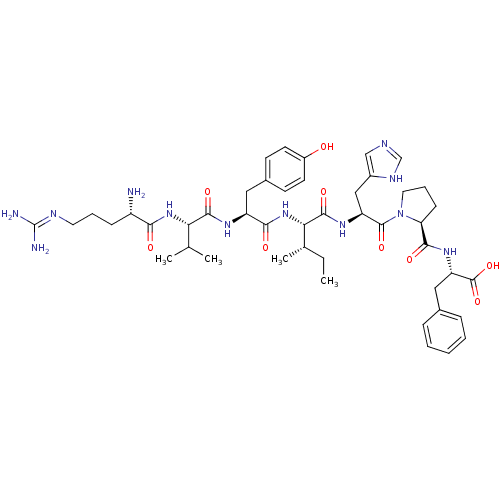
(Ang III | CAS_12687-51-3)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@@H](N)CCCN=C(N)N)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r,wU:39.39,8.17,24.25,52.55,56.58,wD:4.4,2.2,20.21,(2.14,-3.15,;1.3,-1.86,;-.2,-1.94,;-.91,-3.35,;-1.04,-.64,;-2.62,-.74,;-3.32,-2.12,;-2.48,-3.41,;-4.85,-2.21,;-5.68,-.94,;-5,.45,;-5.83,1.73,;-5.14,3.11,;-3.59,3.19,;-2.92,4.55,;-2.77,1.91,;-3.44,.52,;-5.56,-3.58,;-7.1,-3.68,;-7.93,-2.4,;-7.79,-5.05,;-9.33,-5.14,;-10.03,-6.51,;-9.18,-7.82,;-11.56,-6.61,;-12.26,-7.97,;-12.41,-5.33,;-11.71,-3.93,;-12.55,-2.66,;-11.85,-1.26,;-12.71,.05,;-14.25,-.05,;-11.99,1.42,;-6.94,-6.35,;-5.41,-6.27,;-7.65,-7.73,;-.37,.69,;-1.2,1.95,;1.17,.78,;1.9,2.15,;1.06,3.43,;1.27,4.98,;2.66,5.69,;2.37,7.22,;.85,7.44,;.16,6.04,;3.43,2.23,;4.29,.93,;4.14,3.61,;3.31,4.85,;4.43,5.96,;5.8,5.15,;5.62,3.69,;6.71,2.6,;8.11,3.15,;6.47,1.08,;7.67,.11,;9.08,.65,;10.29,-.31,;11.74,.25,;12.94,-.72,;12.71,-2.26,;11.27,-2.8,;10.06,-1.84,;7.44,-1.42,;8.64,-2.4,;6.01,-1.98,)| Show InChI InChI=1S/C46H66N12O9/c1-5-27(4)38(57-40(61)33(21-29-15-17-31(59)18-16-29)53-42(63)37(26(2)3)56-39(60)32(47)13-9-19-51-46(48)49)43(64)54-34(23-30-24-50-25-52-30)44(65)58-20-10-14-36(58)41(62)55-35(45(66)67)22-28-11-7-6-8-12-28/h6-8,11-12,15-18,24-27,32-38,59H,5,9-10,13-14,19-23,47H2,1-4H3,(H,50,52)(H,53,63)(H,54,64)(H,55,62)(H,56,60)(H,57,61)(H,66,67)(H4,48,49,51)/t27-,32-,33-,34-,35-,36-,37-,38-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 36.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 291: 1242-9 (1999)
BindingDB Entry DOI: 10.7270/Q2377783 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(BOVINE) | BDBM85557

(Ang III | CAS_12687-51-3)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@@H](N)CCCN=C(N)N)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r,wU:39.39,8.17,24.25,52.55,56.58,wD:4.4,2.2,20.21,(2.14,-3.15,;1.3,-1.86,;-.2,-1.94,;-.91,-3.35,;-1.04,-.64,;-2.62,-.74,;-3.32,-2.12,;-2.48,-3.41,;-4.85,-2.21,;-5.68,-.94,;-5,.45,;-5.83,1.73,;-5.14,3.11,;-3.59,3.19,;-2.92,4.55,;-2.77,1.91,;-3.44,.52,;-5.56,-3.58,;-7.1,-3.68,;-7.93,-2.4,;-7.79,-5.05,;-9.33,-5.14,;-10.03,-6.51,;-9.18,-7.82,;-11.56,-6.61,;-12.26,-7.97,;-12.41,-5.33,;-11.71,-3.93,;-12.55,-2.66,;-11.85,-1.26,;-12.71,.05,;-14.25,-.05,;-11.99,1.42,;-6.94,-6.35,;-5.41,-6.27,;-7.65,-7.73,;-.37,.69,;-1.2,1.95,;1.17,.78,;1.9,2.15,;1.06,3.43,;1.27,4.98,;2.66,5.69,;2.37,7.22,;.85,7.44,;.16,6.04,;3.43,2.23,;4.29,.93,;4.14,3.61,;3.31,4.85,;4.43,5.96,;5.8,5.15,;5.62,3.69,;6.71,2.6,;8.11,3.15,;6.47,1.08,;7.67,.11,;9.08,.65,;10.29,-.31,;11.74,.25,;12.94,-.72,;12.71,-2.26,;11.27,-2.8,;10.06,-1.84,;7.44,-1.42,;8.64,-2.4,;6.01,-1.98,)| Show InChI InChI=1S/C46H66N12O9/c1-5-27(4)38(57-40(61)33(21-29-15-17-31(59)18-16-29)53-42(63)37(26(2)3)56-39(60)32(47)13-9-19-51-46(48)49)43(64)54-34(23-30-24-50-25-52-30)44(65)58-20-10-14-36(58)41(62)55-35(45(66)67)22-28-11-7-6-8-12-28/h6-8,11-12,15-18,24-27,32-38,59H,5,9-10,13-14,19-23,47H2,1-4H3,(H,50,52)(H,53,63)(H,54,64)(H,55,62)(H,56,60)(H,57,61)(H,66,67)(H4,48,49,51)/t27-,32-,33-,34-,35-,36-,37-,38-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 63.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 291: 1242-9 (1999)
BindingDB Entry DOI: 10.7270/Q2377783 |
More data for this
Ligand-Target Pair | |
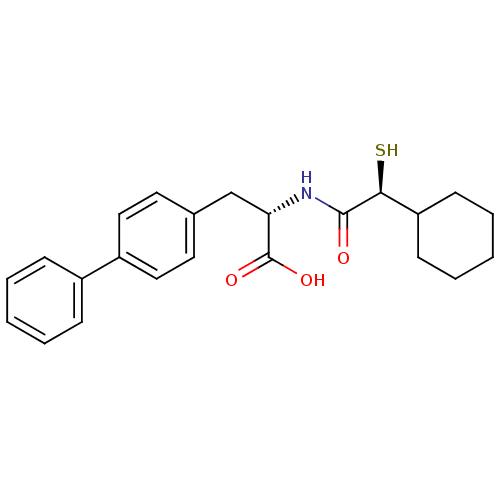
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50411727
(CHEMBL257026)Show SMILES OC(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@@H](S)C1CCCCC1 Show InChI InChI=1S/C23H27NO3S/c25-22(21(28)19-9-5-2-6-10-19)24-20(23(26)27)15-16-11-13-18(14-12-16)17-7-3-1-4-8-17/h1,3-4,7-8,11-14,19-21,28H,2,5-6,9-10,15H2,(H,24,25)(H,26,27)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50411726
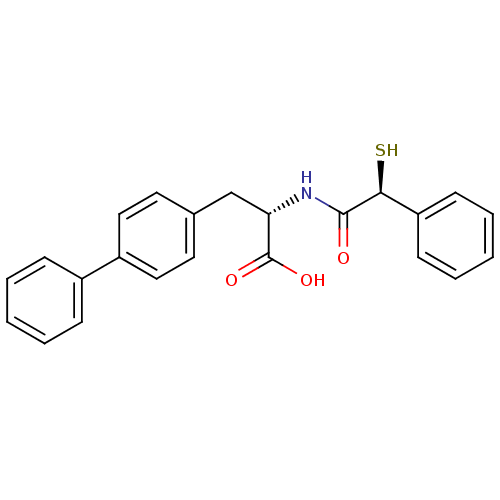
(CHEMBL437595)Show SMILES OC(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@@H](S)c1ccccc1 Show InChI InChI=1S/C23H21NO3S/c25-22(21(28)19-9-5-2-6-10-19)24-20(23(26)27)15-16-11-13-18(14-12-16)17-7-3-1-4-8-17/h1-14,20-21,28H,15H2,(H,24,25)(H,26,27)/t20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50286715
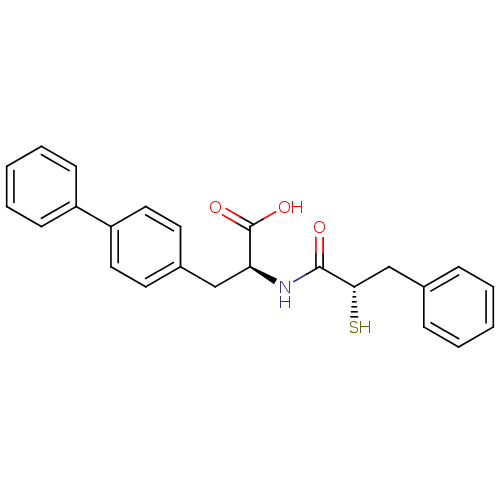
((S)-3-biphenyl-4-yl-2-((S)-2-mercapto-3-phenyl-pro...)Show SMILES OC(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@@H](S)Cc1ccccc1 Show InChI InChI=1S/C24H23NO3S/c26-23(22(29)16-17-7-3-1-4-8-17)25-21(24(27)28)15-18-11-13-20(14-12-18)19-9-5-2-6-10-19/h1-14,21-22,29H,15-16H2,(H,25,26)(H,27,28)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(BOVINE) | BDBM85544
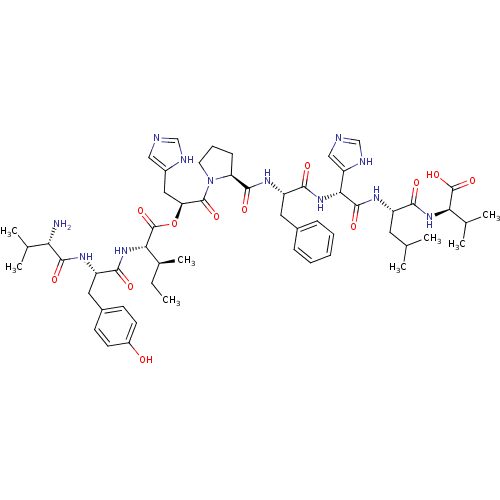
(Ang(3-11))Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)C(C)C)C(=O)O[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(C)C)C(O)=O)c1cnc[nH]1 |r| Show InChI InChI=1S/C56H78N12O12/c1-9-33(8)46(66-49(71)40(63-52(74)44(57)31(4)5)24-35-17-19-37(69)20-18-35)56(79)80-43(25-36-26-58-28-60-36)54(76)68-21-13-16-42(68)51(73)62-39(23-34-14-11-10-12-15-34)50(72)67-47(41-27-59-29-61-41)53(75)64-38(22-30(2)3)48(70)65-45(32(6)7)55(77)78/h10-12,14-15,17-20,26-33,38-40,42-47,69H,9,13,16,21-25,57H2,1-8H3,(H,58,60)(H,59,61)(H,62,73)(H,63,74)(H,64,75)(H,65,70)(H,66,71)(H,67,72)(H,77,78)/t33-,38-,39-,40-,42-,43-,44-,45+,46-,47+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 291: 1242-9 (1999)
BindingDB Entry DOI: 10.7270/Q2377783 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21475
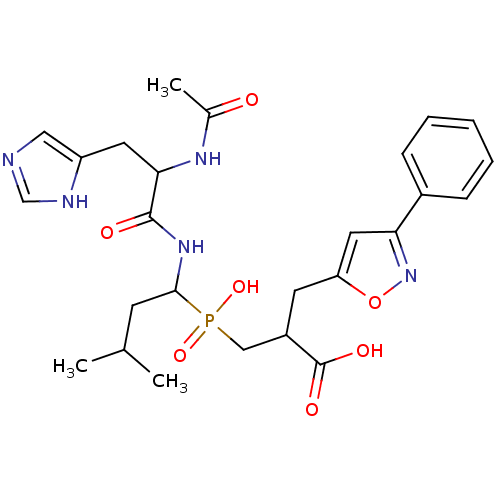
(3-({1-[2-acetamido-3-(1H-imidazol-4-yl)propanamido...)Show SMILES CC(C)CC(NC(=O)C(Cc1cnc[nH]1)NC(C)=O)P(O)(=O)CC(Cc1cc(no1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C26H34N5O7P/c1-16(2)9-24(30-25(33)23(29-17(3)32)11-20-13-27-15-28-20)39(36,37)14-19(26(34)35)10-21-12-22(31-38-21)18-7-5-4-6-8-18/h4-8,12-13,15-16,19,23-24H,9-11,14H2,1-3H3,(H,27,28)(H,29,32)(H,30,33)(H,34,35)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 220 | -38.0 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens
| Assay Description
Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... |
J Med Chem 51: 2216-2226 (2008)
Article DOI: 10.1021/jm701275z
BindingDB Entry DOI: 10.7270/Q2QF8R4J |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21454
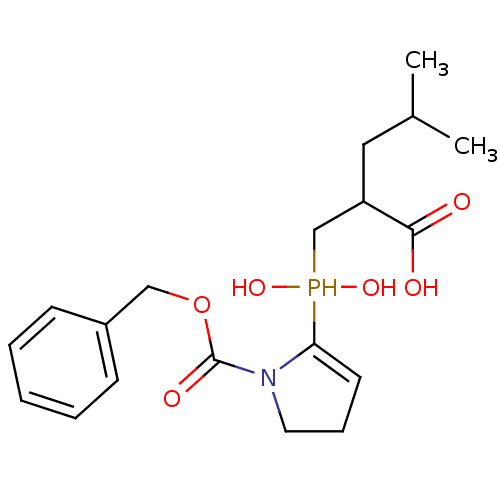
(2-[({1-[(benzyloxy)carbonyl]pyrrolidin-2-yl}(hydro...)Show SMILES CC(C)CC(CP(O)(O)C1=CCCN1C(=O)OCc1ccccc1)C(O)=O |t:9| Show InChI InChI=1S/C19H28NO6P/c1-14(2)11-16(18(21)22)13-27(24,25)17-9-6-10-20(17)19(23)26-12-15-7-4-3-5-8-15/h3-5,7-9,14,16,24-25,27H,6,10-13H2,1-2H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | -37.2 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens
| Assay Description
Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... |
J Med Chem 51: 2216-2226 (2008)
Article DOI: 10.1021/jm701275z
BindingDB Entry DOI: 10.7270/Q2QF8R4J |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21452
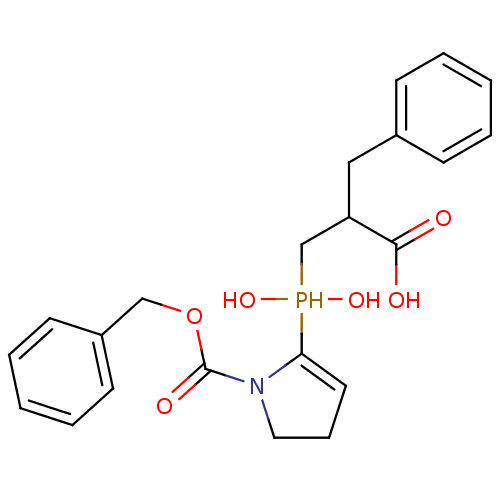
(2-benzyl-3-({1-[(benzyloxy)carbonyl]pyrrolidin-2-y...)Show SMILES OC(=O)C(Cc1ccccc1)CP(O)(O)C1=CCCN1C(=O)OCc1ccccc1 |t:16| Show InChI InChI=1S/C22H26NO6P/c24-21(25)19(14-17-8-3-1-4-9-17)16-30(27,28)20-12-7-13-23(20)22(26)29-15-18-10-5-2-6-11-18/h1-6,8-12,19,27-28,30H,7,13-16H2,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | -37.2 | n/a | n/a | n/a | n/a | n/a | 6.8 | 25 |
University of Athens
| Assay Description
Continuous assays were performed by recording the fluorescence increase at 405 nm (excitation@ 320 nm) induced by the cleavage of fluorogenic substra... |
J Med Chem 51: 2216-2226 (2008)
Article DOI: 10.1021/jm701275z
BindingDB Entry DOI: 10.7270/Q2QF8R4J |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50411722
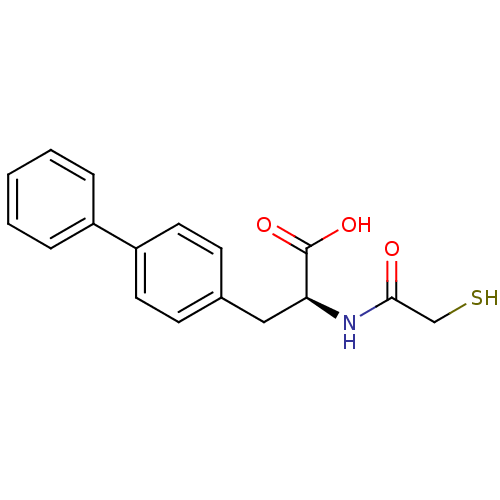
(CHEMBL258333)Show InChI InChI=1S/C17H17NO3S/c19-16(11-22)18-15(17(20)21)10-12-6-8-14(9-7-12)13-4-2-1-3-5-13/h1-9,15,22H,10-11H2,(H,18,19)(H,20,21)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(BOVINE) | BDBM82077
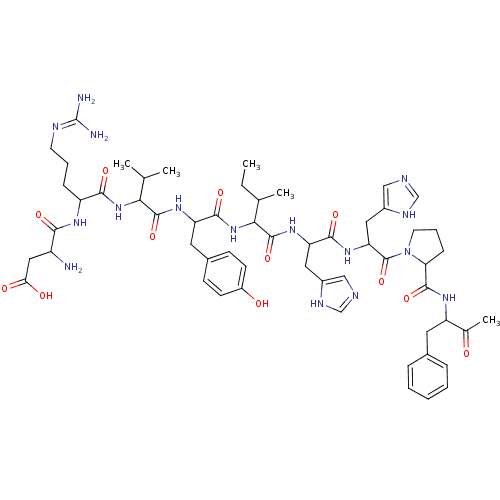
(Angiotensin II | CAS_11128-99-7 | NSC_439662)Show SMILES CCC(C)C(NC(=O)C(Cc1ccc(O)cc1)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)C(N)CC(O)=O)C(C)C)C(=O)NC(Cc1cnc[nH]1)C(=O)NC(Cc1cnc[nH]1)C(=O)N1CCCC1C(=O)NC(Cc1ccccc1)C(C)=O |(1.13,.52,;1.58,1.99,;.53,3.12,;-.97,2.77,;.98,4.59,;2.48,4.94,;3.54,3.81,;3.08,2.34,;5.04,4.16,;5.49,5.63,;4.44,6.76,;2.93,6.41,;1.88,7.54,;2.33,9.01,;1.28,10.14,;3.84,9.36,;4.89,8.23,;6.09,3.03,;7.59,3.38,;8.04,4.85,;8.64,2.25,;10.14,2.6,;11.19,1.47,;10.74,-0,;12.69,1.82,;13.74,.69,;13.29,-.78,;14.34,-1.91,;13.89,-3.38,;12.39,-3.73,;11.94,-5.2,;11.34,-2.6,;13.14,3.29,;14.64,3.64,;15.69,2.51,;15.09,5.11,;14.04,6.24,;16.59,5.46,;17.04,6.93,;15.99,8.06,;18.54,7.28,;8.19,.78,;9.24,-.35,;6.69,.43,;-.07,5.72,;.38,7.19,;-1.57,5.37,;-2.62,6.5,;-2.17,7.97,;-3.22,9.1,;-2.92,10.61,;-4.27,11.35,;-5.4,10.3,;-4.75,8.91,;-4.12,6.15,;-4.57,4.68,;-5.17,7.28,;-6.67,6.93,;-6.64,5.39,;-7.96,4.6,;-9.38,5.2,;-10.39,4.04,;-9.6,2.72,;-8.1,3.06,;-7.72,8.06,;-7.27,9.53,;-9.22,7.71,;-10.38,8.72,;-11.7,7.93,;-11.36,6.43,;-9.82,6.29,;-10.42,4.87,;-10.87,3.4,;-11.95,4.69,;-12.55,3.27,;-14.08,3.08,;-14.68,1.66,;-13.76,.43,;-14.36,-.98,;-15.89,-1.17,;-16.81,.06,;-16.21,1.48,;-11.63,2.04,;-10.1,2.23,;-12.23,.62,)| Show InChI InChI=1S/C57H80N16O12/c1-6-32(4)48(72-52(81)42(23-35-16-18-38(75)19-17-35)68-54(83)47(31(2)3)71-50(79)40(14-10-20-63-57(59)60)66-49(78)39(58)26-46(76)77)55(84)69-43(24-36-27-61-29-64-36)51(80)70-44(25-37-28-62-30-65-37)56(85)73-21-11-15-45(73)53(82)67-41(33(5)74)22-34-12-8-7-9-13-34/h7-9,12-13,16-19,27-32,39-45,47-48,75H,6,10-11,14-15,20-26,58H2,1-5H3,(H,61,64)(H,62,65)(H,66,78)(H,67,82)(H,68,83)(H,69,84)(H,70,80)(H,71,79)(H,72,81)(H,76,77)(H4,59,60,63) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 411 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 291: 1242-9 (1999)
BindingDB Entry DOI: 10.7270/Q2377783 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50411724
(CHEMBL271224)Show SMILES OC(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)[C@@H](S)CC1CCCCC1 Show InChI InChI=1S/C24H29NO3S/c26-23(22(29)16-17-7-3-1-4-8-17)25-21(24(27)28)15-18-11-13-20(14-12-18)19-9-5-2-6-10-19/h2,5-6,9-14,17,21-22,29H,1,3-4,7-8,15-16H2,(H,25,26)(H,27,28)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2 by fluorescence assay |
Bioorg Med Chem Lett 18: 732-7 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.048
BindingDB Entry DOI: 10.7270/Q2GT5PCB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data