

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50501643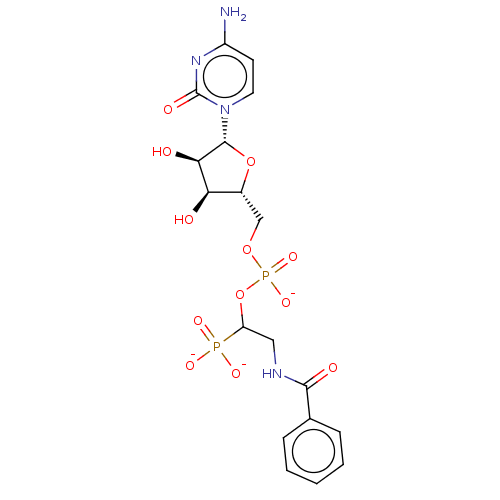 (CHEMBL4065191) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC method | J Med Chem 60: 2135-2141 (2017) Article DOI: 10.1021/acs.jmedchem.6b01644 BindingDB Entry DOI: 10.7270/Q2XP77Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50501643 (CHEMBL4065191) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC method | J Med Chem 60: 2135-2141 (2017) Article DOI: 10.1021/acs.jmedchem.6b01644 BindingDB Entry DOI: 10.7270/Q2XP77Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50590206 (CHEMBL4117853) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00079a BindingDB Entry DOI: 10.7270/Q2183BHP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50501640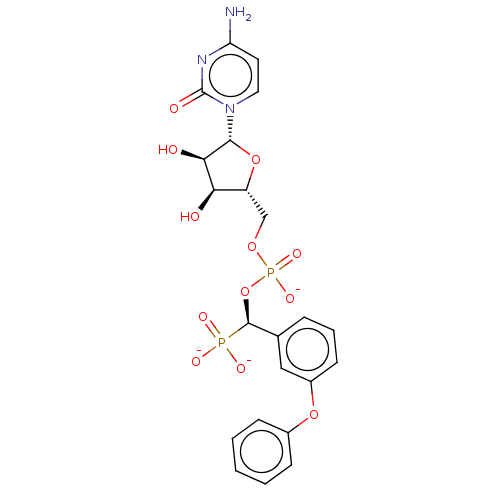 (CHEMBL4102509) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC method | J Med Chem 60: 2135-2141 (2017) Article DOI: 10.1021/acs.jmedchem.6b01644 BindingDB Entry DOI: 10.7270/Q2XP77Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50127702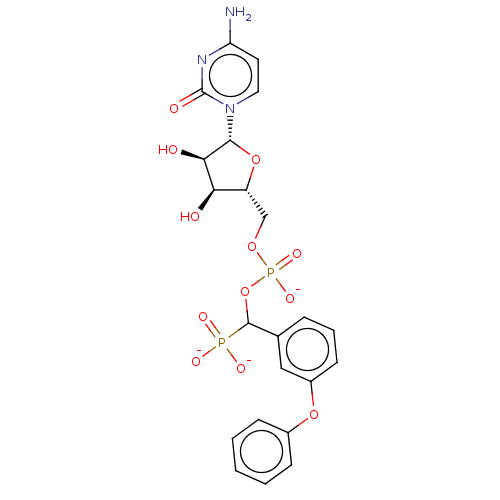 (CHEMBL3629697) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant ST6Gal-1 using CMP-Neu5Ac and p-nitrophenyl LacNAc as donar and acceptor by Lineweaver-Burk double recipr... | J Med Chem 58: 7972-90 (2015) Article DOI: 10.1021/acs.jmedchem.5b01181 BindingDB Entry DOI: 10.7270/Q20V8FMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50501638 (CHEMBL4091389) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC method | J Med Chem 60: 2135-2141 (2017) Article DOI: 10.1021/acs.jmedchem.6b01644 BindingDB Entry DOI: 10.7270/Q2XP77Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50501638 (CHEMBL4091389) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC method | J Med Chem 60: 2135-2141 (2017) Article DOI: 10.1021/acs.jmedchem.6b01644 BindingDB Entry DOI: 10.7270/Q2XP77Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50127703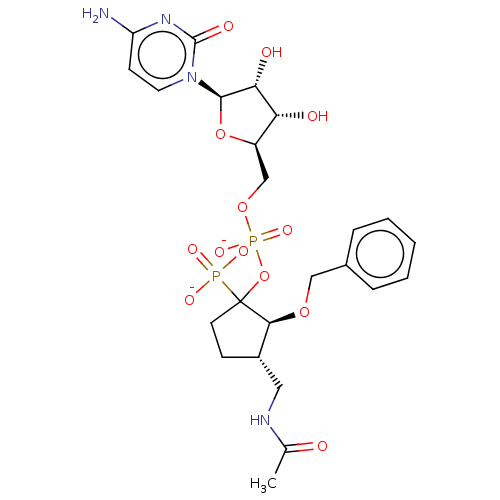 (CHEMBL3629696) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant ST6Gal-1 using CMP-Neu5Ac and p-nitrophenyl LacNAc as donar and acceptor by Lineweaver-Burk double recipr... | J Med Chem 58: 7972-90 (2015) Article DOI: 10.1021/acs.jmedchem.5b01181 BindingDB Entry DOI: 10.7270/Q20V8FMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50127703 (CHEMBL3629696) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC method | J Med Chem 60: 2135-2141 (2017) Article DOI: 10.1021/acs.jmedchem.6b01644 BindingDB Entry DOI: 10.7270/Q2XP77Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50127703 (CHEMBL3629696) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant ST6Gal-1 using CMP-Neu5Ac and p-nitrophenyl LacNAc as donar and acceptor by Lineweaver-Burk double recipr... | J Med Chem 58: 7972-90 (2015) Article DOI: 10.1021/acs.jmedchem.5b01181 BindingDB Entry DOI: 10.7270/Q20V8FMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50127703 (CHEMBL3629696) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC method | J Med Chem 60: 2135-2141 (2017) Article DOI: 10.1021/acs.jmedchem.6b01644 BindingDB Entry DOI: 10.7270/Q2XP77Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Rattus norvegicus) | BDBM50559955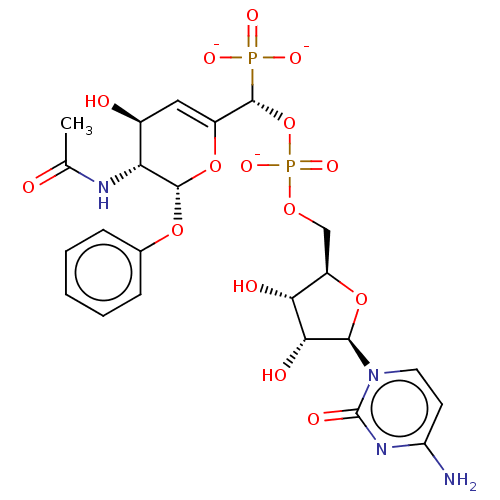 (CHEMBL4776007) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat liver ST6Gal-1 by HPLC-based assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115561 BindingDB Entry DOI: 10.7270/Q25M69DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50501642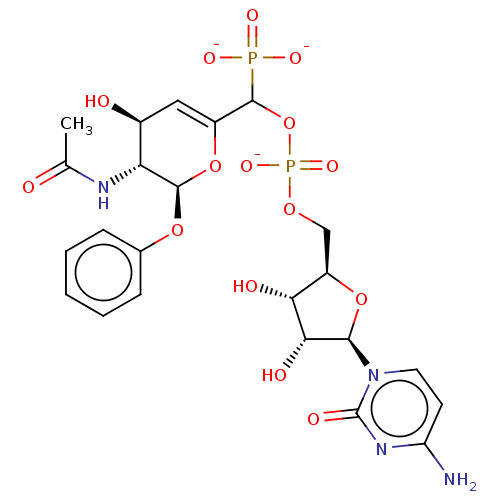 (CHEMBL3629698) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC method | J Med Chem 60: 2135-2141 (2017) Article DOI: 10.1021/acs.jmedchem.6b01644 BindingDB Entry DOI: 10.7270/Q2XP77Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50501642 (CHEMBL3629698) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC method | J Med Chem 60: 2135-2141 (2017) Article DOI: 10.1021/acs.jmedchem.6b01644 BindingDB Entry DOI: 10.7270/Q2XP77Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50501637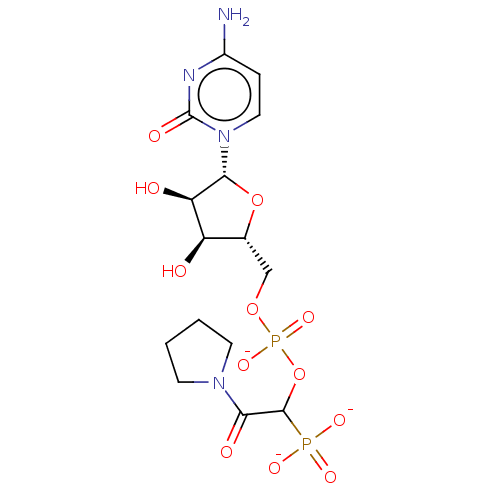 (CHEMBL4063545) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC method | J Med Chem 60: 2135-2141 (2017) Article DOI: 10.1021/acs.jmedchem.6b01644 BindingDB Entry DOI: 10.7270/Q2XP77Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50501637 (CHEMBL4063545) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC method | J Med Chem 60: 2135-2141 (2017) Article DOI: 10.1021/acs.jmedchem.6b01644 BindingDB Entry DOI: 10.7270/Q2XP77Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Rattus norvegicus) | BDBM50501640 (CHEMBL4102509) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat liver ST6Gal-1 using p-nitrophenyl-DL-alanine measured up to 20 mins by HPLC analysis | Citation and Details Article DOI: 10.1016/j.bmc.2020.115561 BindingDB Entry DOI: 10.7270/Q25M69DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50501644 (CHEMBL4097206) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC method | J Med Chem 60: 2135-2141 (2017) Article DOI: 10.1021/acs.jmedchem.6b01644 BindingDB Entry DOI: 10.7270/Q2XP77Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50501644 (CHEMBL4097206) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC method | J Med Chem 60: 2135-2141 (2017) Article DOI: 10.1021/acs.jmedchem.6b01644 BindingDB Entry DOI: 10.7270/Q2XP77Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50501641 (CHEMBL4081024) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC method | J Med Chem 60: 2135-2141 (2017) Article DOI: 10.1021/acs.jmedchem.6b01644 BindingDB Entry DOI: 10.7270/Q2XP77Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50501641 (CHEMBL4081024) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC method | J Med Chem 60: 2135-2141 (2017) Article DOI: 10.1021/acs.jmedchem.6b01644 BindingDB Entry DOI: 10.7270/Q2XP77Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50501639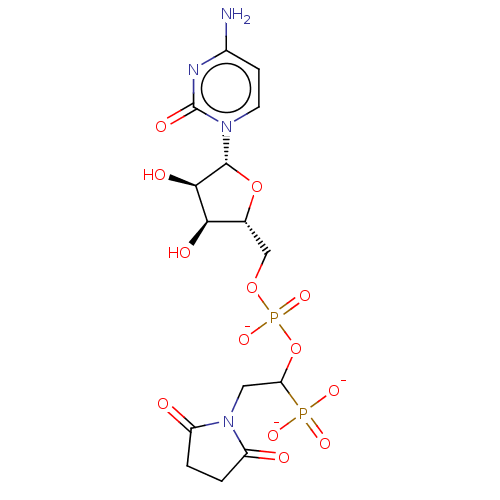 (CHEMBL4083608) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC method | J Med Chem 60: 2135-2141 (2017) Article DOI: 10.1021/acs.jmedchem.6b01644 BindingDB Entry DOI: 10.7270/Q2XP77Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50127706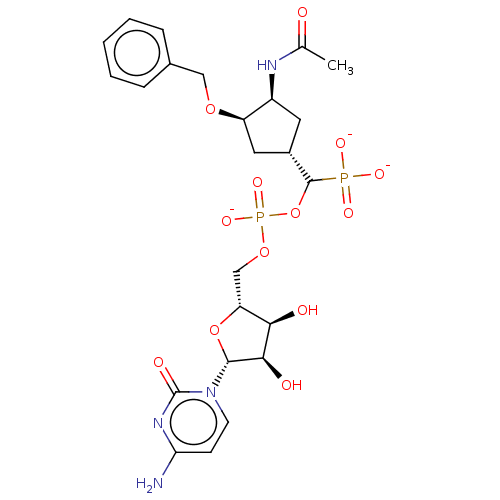 (CHEMBL3629692) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 436 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant ST6Gal-1 using CMP-Neu5Ac and p-nitrophenyl LacNAc as donar and acceptor by Lineweaver-Burk double recipr... | J Med Chem 58: 7972-90 (2015) Article DOI: 10.1021/acs.jmedchem.5b01181 BindingDB Entry DOI: 10.7270/Q20V8FMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50127706 (CHEMBL3629692) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 436 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant ST6Gal-1 using CMP-Neu5Ac and p-nitrophenyl LacNAc as donar and acceptor by Lineweaver-Burk double recipr... | J Med Chem 58: 7972-90 (2015) Article DOI: 10.1021/acs.jmedchem.5b01181 BindingDB Entry DOI: 10.7270/Q20V8FMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50127704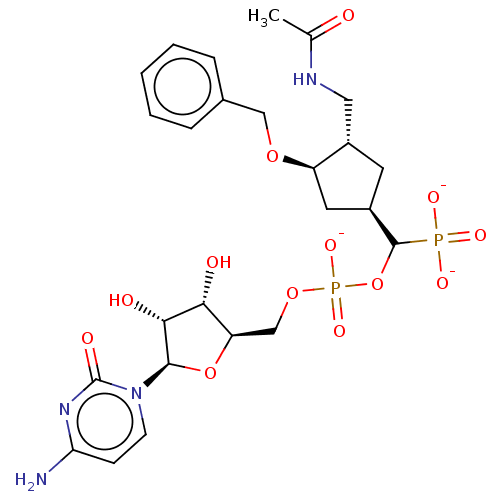 (CHEMBL3629694) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 865 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant ST6Gal-1 using CMP-Neu5Ac and p-nitrophenyl LacNAc as donar and acceptor by Lineweaver-Burk double recipr... | J Med Chem 58: 7972-90 (2015) Article DOI: 10.1021/acs.jmedchem.5b01181 BindingDB Entry DOI: 10.7270/Q20V8FMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50127705 (CHEMBL3629693) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant ST6Gal-1 using CMP-Neu5Ac and p-nitrophenyl LacNAc as donar and acceptor by Lineweaver-Burk double recipr... | J Med Chem 58: 7972-90 (2015) Article DOI: 10.1021/acs.jmedchem.5b01181 BindingDB Entry DOI: 10.7270/Q20V8FMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50127707 (CHEMBL3629691) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant ST6Gal-1 using CMP-Neu5Ac and p-nitrophenyl LacNAc as donar and acceptor by Lineweaver-Burk double recipr... | J Med Chem 58: 7972-90 (2015) Article DOI: 10.1021/acs.jmedchem.5b01181 BindingDB Entry DOI: 10.7270/Q20V8FMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50127709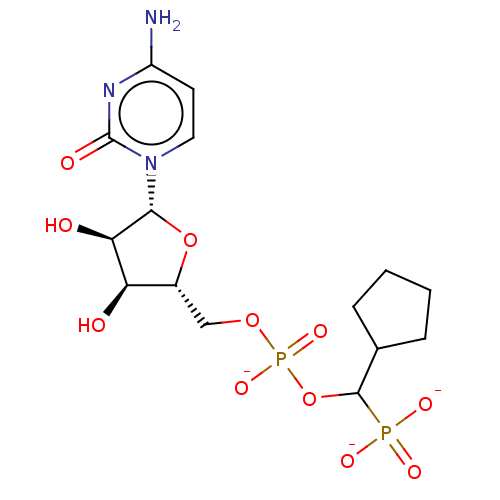 (CHEMBL3629689) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant ST6Gal-1 using CMP-Neu5Ac and p-nitrophenyl LacNAc as donar and acceptor by Lineweaver-Burk double recipr... | J Med Chem 58: 7972-90 (2015) Article DOI: 10.1021/acs.jmedchem.5b01181 BindingDB Entry DOI: 10.7270/Q20V8FMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50127709 (CHEMBL3629689) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC method | J Med Chem 60: 2135-2141 (2017) Article DOI: 10.1021/acs.jmedchem.6b01644 BindingDB Entry DOI: 10.7270/Q2XP77Z0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50590208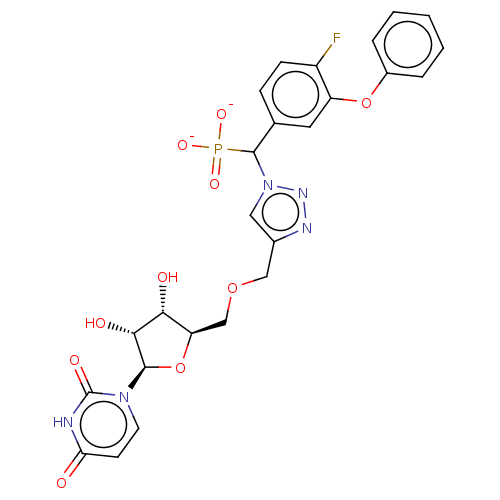 (CHEMBL5185498) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00079a BindingDB Entry DOI: 10.7270/Q2183BHP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50590208 (CHEMBL5185498) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00079a BindingDB Entry DOI: 10.7270/Q2183BHP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50590209 (CHEMBL5175639) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00079a BindingDB Entry DOI: 10.7270/Q2183BHP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50590209 (CHEMBL5175639) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00079a BindingDB Entry DOI: 10.7270/Q2183BHP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50559954 (CHEMBL4755740) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal 6His-tagged ST6Gal-1 (Glu44 to Cys406 residues) | Citation and Details Article DOI: 10.1016/j.bmc.2020.115561 BindingDB Entry DOI: 10.7270/Q25M69DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50559951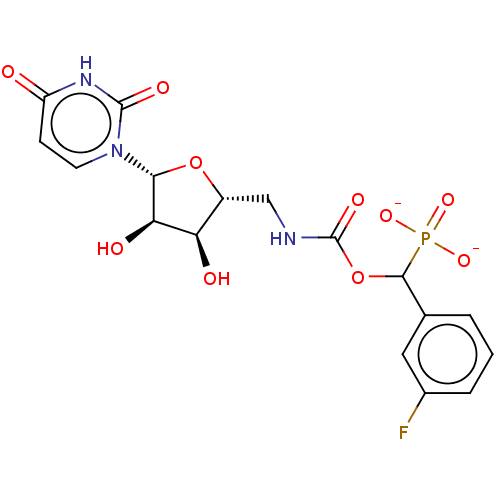 (CHEMBL4751247) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal 6His-tagged ST6Gal-1 (Glu44 to Cys406 residues) | Citation and Details Article DOI: 10.1016/j.bmc.2020.115561 BindingDB Entry DOI: 10.7270/Q25M69DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50559952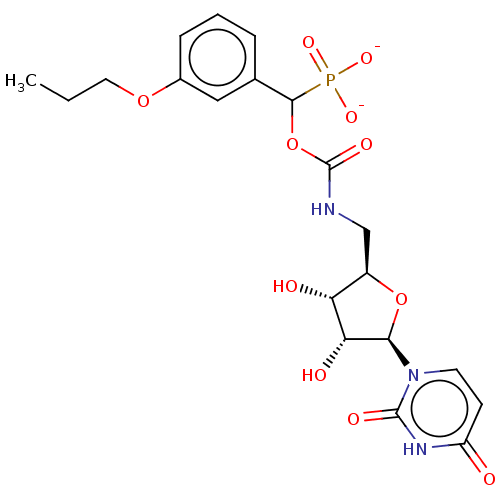 (CHEMBL4743892) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal 6His-tagged ST6Gal-1 (Glu44 to Cys406 residues) | Citation and Details Article DOI: 10.1016/j.bmc.2020.115561 BindingDB Entry DOI: 10.7270/Q25M69DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50590207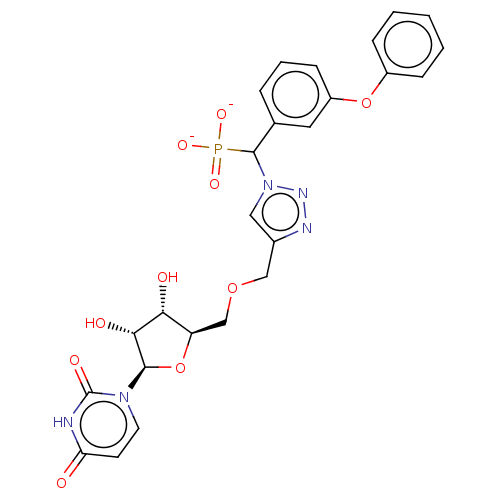 (CHEMBL5180951) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00079a BindingDB Entry DOI: 10.7270/Q2183BHP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50590207 (CHEMBL5180951) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00079a BindingDB Entry DOI: 10.7270/Q2183BHP | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50559953 (CHEMBL4757654) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal 6His-tagged ST6Gal-1 (Glu44 to Cys406 residues) | Citation and Details Article DOI: 10.1016/j.bmc.2020.115561 BindingDB Entry DOI: 10.7270/Q25M69DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50559953 (CHEMBL4757654) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal 6His-tagged ST6Gal-1 (Glu44 to Cys406 residues) | Citation and Details Article DOI: 10.1016/j.bmc.2020.115561 BindingDB Entry DOI: 10.7270/Q25M69DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Rattus norvegicus) | BDBM50310540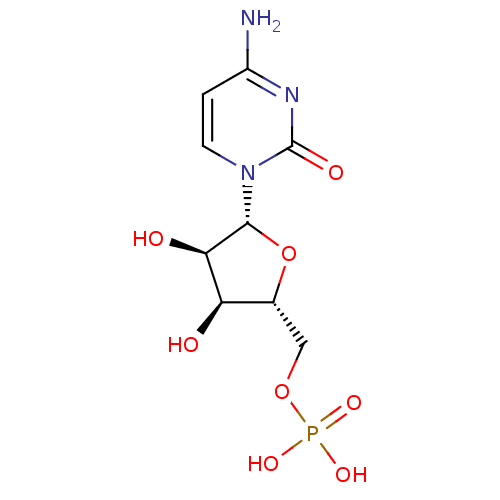 (((2R,3S,4R,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Inhibitory activity evaluated against alpha-2,6-sialyltransferase from rat liver | Bioorg Med Chem Lett 13: 301-4 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2HBM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50559950 (CHEMBL4745184) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal 6His-tagged ST6Gal-1 (Glu44 to Cys406 residues) | Citation and Details Article DOI: 10.1016/j.bmc.2020.115561 BindingDB Entry DOI: 10.7270/Q25M69DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50559950 (CHEMBL4745184) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal 6His-tagged ST6Gal-1 (Glu44 to Cys406 residues) | Citation and Details Article DOI: 10.1016/j.bmc.2020.115561 BindingDB Entry DOI: 10.7270/Q25M69DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Rattus norvegicus) | BDBM50366837 (CHEMBL608619) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Inhibitory activity evaluated against alpha-2,6-sialyltransferase from rat liver | Bioorg Med Chem Lett 13: 301-4 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Rattus norvegicus) | BDBM50366833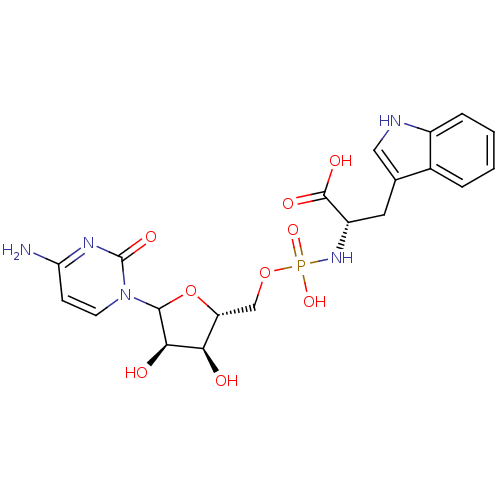 (CHEMBL608928) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Inhibitory activity evaluated against alpha-2,6-sialyltransferase from rat liver | Bioorg Med Chem Lett 13: 301-4 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Rattus norvegicus) | BDBM50366836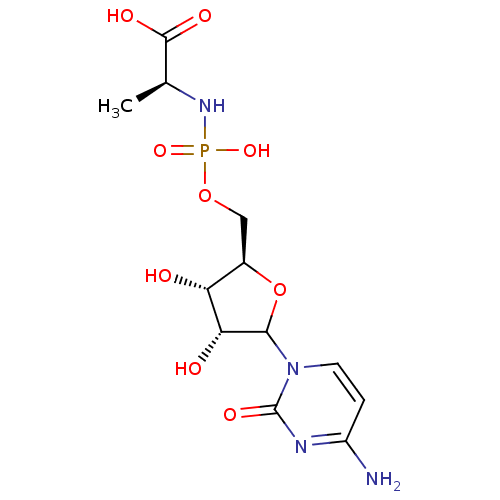 (CHEMBL610554) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Inhibitory activity evaluated against alpha-2,6-sialyltransferase from rat liver | Bioorg Med Chem Lett 13: 301-4 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Rattus norvegicus) | BDBM50366835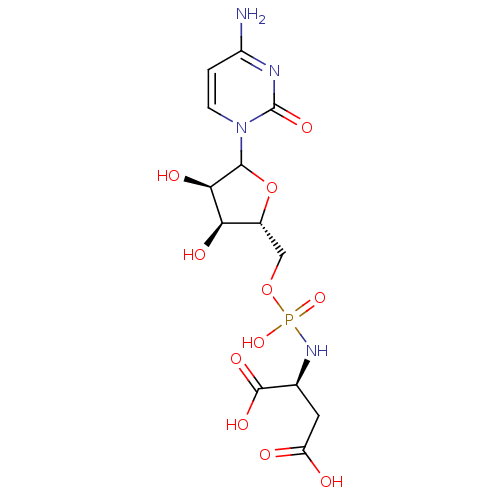 (CHEMBL609514) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Inhibitory activity evaluated against alpha-2,6-sialyltransferase from rat liver | Bioorg Med Chem Lett 13: 301-4 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Rattus norvegicus) | BDBM50366838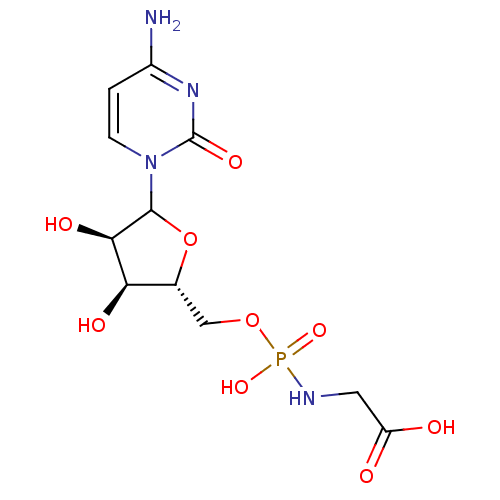 (CHEMBL609215) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Inhibitory activity evaluated against alpha-2,6-sialyltransferase from rat liver | Bioorg Med Chem Lett 13: 301-4 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Rattus norvegicus) | BDBM50366832 (CHEMBL609516) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Inhibitory activity evaluated against alpha-2,6-sialyltransferase from rat liver | Bioorg Med Chem Lett 13: 301-4 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Rattus norvegicus) | BDBM50366834 (CHEMBL609800) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Inhibitory activity evaluated against alpha-2,6-sialyltransferase from rat liver | Bioorg Med Chem Lett 13: 301-4 (2002) BindingDB Entry DOI: 10.7270/Q2FJ2HBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 69 total ) | Next | Last >> |