 Found 624 hits Enz. Inhib. hit(s) with Target = 'Cholecystokinin B'
Found 624 hits Enz. Inhib. hit(s) with Target = 'Cholecystokinin B' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholecystokinin
(GUINEA PIG) | BDBM81963
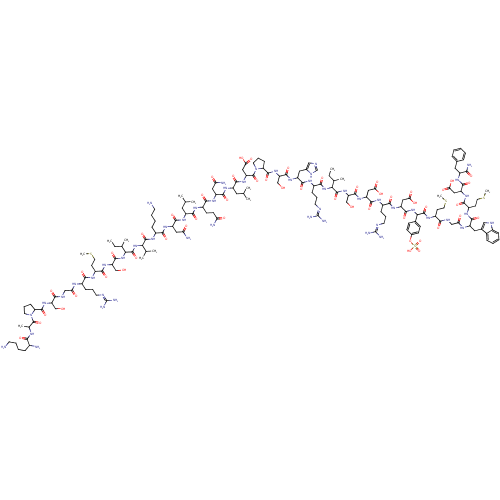
(CAS_9011-97-6 | CCK | CCK33 | CHOLECYSTOKININ)Show SMILES CCC(C)C(NC(=O)C(CCCNC(N)=N)NC(=O)C(Cc1c[nH]cn1)NC(=O)C(CO)NC(=O)C1CCCN1C(=O)C(CC(O)=O)NC(=O)C(CC(C)C)NC(=O)C(CC(N)=O)NC(=O)C(CCC(N)=O)NC(=O)C(CC(C)C)NC(=O)C(CC(N)=O)NC(=O)C(CCCCN)NC(=O)C(NC(=O)C(NC(=O)C(CO)NC(=O)C(CCSC)NC(=O)C(CCCNC(N)=N)NC(=O)CNC(=O)C(CO)NC(=O)C1CCCN1C(=O)C(C)NC(=O)C(N)CCCCN)C(C)CC)C(C)C)C(=O)NC(CO)C(=O)NC(CC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NC(CC(O)=O)C(=O)NC(C(=O)NC(CCSC)C(=O)NCC(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCSC)C(=O)NC(CC(O)=O)C(=O)NC(Cc1ccccc1)C(N)=O)c1ccc(OS(O)(=O)=O)cc1 Show InChI InChI=1S/C166H261N51O52S4/c1-15-84(9)130(159(261)211-116(78-220)154(256)206-110(68-125(227)228)150(252)191-97(38-27-54-182-165(176)177)139(241)205-112(70-127(231)232)152(254)215-132(88-42-44-91(45-43-88)269-273(266,267)268)161(263)197-100(48-58-270-12)135(237)185-75-124(226)190-106(64-89-72-184-94-35-21-20-33-92(89)94)146(248)195-101(49-59-271-13)141(243)204-111(69-126(229)230)151(253)198-103(133(173)235)63-87-31-18-17-19-32-87)213-143(245)98(39-28-55-183-166(178)179)192-147(249)107(65-90-73-180-80-187-90)201-153(255)115(77-219)210-157(259)119-41-30-57-217(119)163(265)113(71-128(233)234)207-145(247)105(62-82(5)6)200-149(251)109(67-122(172)224)203-140(242)99(46-47-120(170)222)193-144(246)104(61-81(3)4)199-148(250)108(66-121(171)223)202-138(240)96(36-23-25-52-168)196-158(260)129(83(7)8)212-160(262)131(85(10)16-2)214-155(257)117(79-221)208-142(244)102(50-60-272-14)194-137(239)95(37-26-53-181-164(174)175)189-123(225)74-186-136(238)114(76-218)209-156(258)118-40-29-56-216(118)162(264)86(11)188-134(236)93(169)34-22-24-51-167/h17-21,31-33,35,42-45,72-73,80-86,93,95-119,129-132,184,218-221H,15-16,22-30,34,36-41,46-71,74-79,167-169H2,1-14H3,(H2,170,222)(H2,171,223)(H2,172,224)(H2,173,235)(H,180,187)(H,185,237)(H,186,238)(H,188,236)(H,189,225)(H,190,226)(H,191,252)(H,192,249)(H,193,246)(H,194,239)(H,195,248)(H,196,260)(H,197,263)(H,198,253)(H,199,250)(H,200,251)(H,201,255)(H,202,240)(H,203,242)(H,204,243)(H,205,241)(H,206,256)(H,207,247)(H,208,244)(H,209,258)(H,210,259)(H,211,261)(H,212,262)(H,213,245)(H,214,257)(H,215,254)(H,227,228)(H,229,230)(H,231,232)(H,233,234)(H4,174,175,181)(H4,176,177,182)(H4,178,179,183)(H,266,267,268) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 162: 273-80 (1989)
Article DOI: 10.1016/0014-2999(89)90290-2
BindingDB Entry DOI: 10.7270/Q2QF8RB7 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50056101
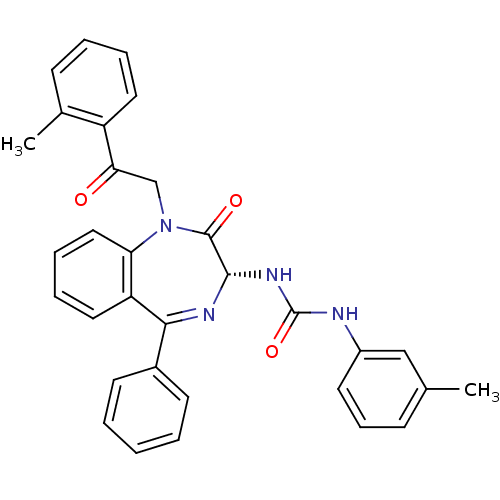
(1-[(R)-2-Oxo-1-(2-oxo-2-o-tolyl-ethyl)-5-phenyl-2,...)Show SMILES Cc1cccc(NC(=O)N[C@@H]2N=C(c3ccccc3)c3ccccc3N(CC(=O)c3ccccc3C)C2=O)c1 |t:11| Show InChI InChI=1S/C32H28N4O3/c1-21-11-10-15-24(19-21)33-32(39)35-30-31(38)36(20-28(37)25-16-7-6-12-22(25)2)27-18-9-8-17-26(27)29(34-30)23-13-4-3-5-14-23/h3-19,30H,20H2,1-2H3,(H2,33,35,39)/t30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 269: 725-31 (1994)
BindingDB Entry DOI: 10.7270/Q2WD3Z2F |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM50005463
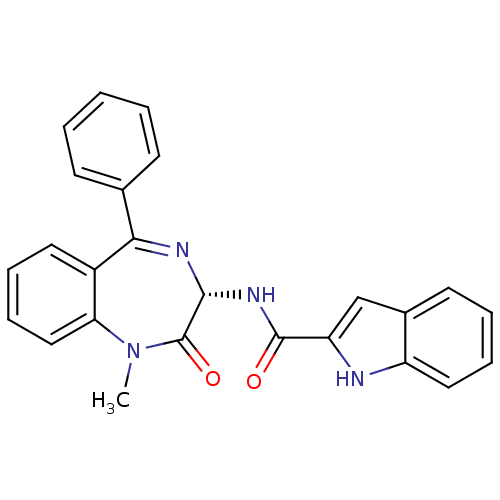
((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)c2cc3ccccc3[nH]2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C25H20N4O2/c1-29-21-14-8-6-12-18(21)22(16-9-3-2-4-10-16)27-23(25(29)31)28-24(30)20-15-17-11-5-7-13-19(17)26-20/h2-15,23,26H,1H3,(H,28,30)/t23-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 162: 273-80 (1989)
Article DOI: 10.1016/0014-2999(89)90290-2
BindingDB Entry DOI: 10.7270/Q2QF8RB7 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 269: 725-31 (1994)
BindingDB Entry DOI: 10.7270/Q2WD3Z2F |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM50040671
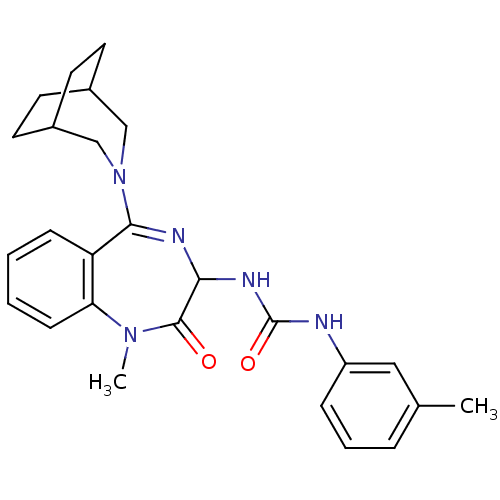
(1-[5-(3-Aza-bicyclo[3.2.2]non-3-yl)-1-methyl-2-oxo...)Show SMILES CN1c2ccccc2C(=NC(NC(=O)Nc2cccc(C)c2)C1=O)N1CC2CCC(CC2)C1 |c:9,(7.22,-6.97,;7.67,-8.45,;6.69,-9.39,;5.36,-8.63,;4.02,-9.39,;4.02,-10.93,;5.36,-11.69,;6.71,-10.96,;7.63,-11.85,;9.14,-11.6,;9.86,-10.19,;11.4,-10.21,;12.13,-11.53,;11.35,-12.86,;13.67,-11.57,;14.48,-10.26,;13.72,-8.91,;14.5,-7.6,;16.04,-7.62,;16.81,-8.95,;18.33,-8.98,;16.01,-10.29,;9.18,-8.72,;10.1,-7.49,;7.17,-13.34,;5.55,-13.5,;4.73,-14.54,;5.14,-16.05,;6.65,-16.64,;8.09,-15.8,;7.22,-14.74,;6.1,-14.2,;8.22,-14.23,)| Show InChI InChI=1S/C26H31N5O2/c1-17-6-5-7-20(14-17)27-26(33)29-23-25(32)30(2)22-9-4-3-8-21(22)24(28-23)31-15-18-10-11-19(16-31)13-12-18/h3-9,14,18-19,23H,10-13,15-16H2,1-2H3,(H2,27,29,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 46: 943-8 (1994)
BindingDB Entry DOI: 10.7270/Q2QR4VMN |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Occupational and Environmental Health
Curated by PDSP Ki Database
| |
Br J Pharmacol 117: 1558-64 (1996)
Article DOI: 10.1111/j.1476-5381.1996.tb15321.x
BindingDB Entry DOI: 10.7270/Q28W3BT3 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50056101

(1-[(R)-2-Oxo-1-(2-oxo-2-o-tolyl-ethyl)-5-phenyl-2,...)Show SMILES Cc1cccc(NC(=O)N[C@@H]2N=C(c3ccccc3)c3ccccc3N(CC(=O)c3ccccc3C)C2=O)c1 |t:11| Show InChI InChI=1S/C32H28N4O3/c1-21-11-10-15-24(19-21)33-32(39)35-30-31(38)36(20-28(37)25-16-7-6-12-22(25)2)27-18-9-8-17-26(27)29(34-30)23-13-4-3-5-14-23/h3-19,30H,20H2,1-2H3,(H2,33,35,39)/t30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 269: 725-31 (1994)
BindingDB Entry DOI: 10.7270/Q2WD3Z2F |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50092393
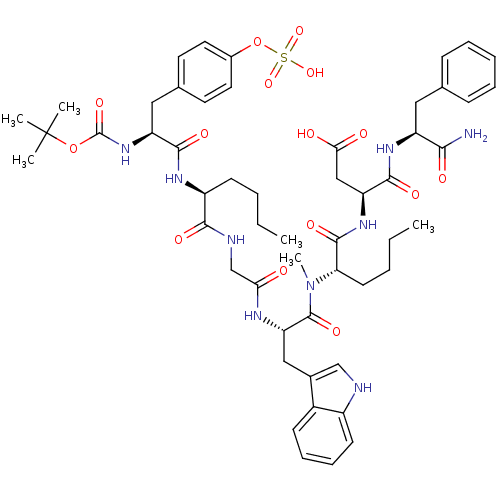
(3-(2-{[2-(2-{2-[2-tert-Butoxycarbonylamino-3-(4-su...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)OC(C)(C)C)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N(C)[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C53H71N9O15S/c1-7-9-19-38(58-48(68)40(61-52(72)76-53(3,4)5)27-33-22-24-35(25-23-33)77-78(73,74)75)47(67)56-31-44(63)57-42(28-34-30-55-37-20-15-14-18-36(34)37)51(71)62(6)43(21-10-8-2)50(70)60-41(29-45(64)65)49(69)59-39(46(54)66)26-32-16-12-11-13-17-32/h11-18,20,22-25,30,38-43,55H,7-10,19,21,26-29,31H2,1-6H3,(H2,54,66)(H,56,67)(H,57,63)(H,58,68)(H,59,69)(H,60,70)(H,61,72)(H,64,65)(H,73,74,75)/t38-,39-,40-,41-,42-,43-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against Cholecystokinin type B receptor expressed in CHO cells on the rat brain. |
J Med Chem 43: 3614-23 (2000)
BindingDB Entry DOI: 10.7270/Q2RN373C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50005463

((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)c2cc3ccccc3[nH]2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C25H20N4O2/c1-29-21-14-8-6-12-18(21)22(16-9-3-2-4-10-16)27-23(25(29)31)28-24(30)20-15-17-11-5-7-13-19(17)26-20/h2-15,23,26H,1H3,(H,28,30)/t23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
Eur J Pharmacol 232: 13-9 (1993)
Article DOI: 10.1016/0014-2999(93)90722-t
BindingDB Entry DOI: 10.7270/Q2P26WN2 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50084033
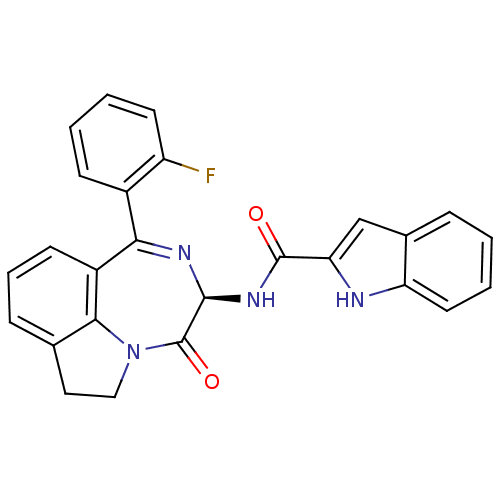
(1H-Indole-2-carboxylic acid [(R)-1-(2-fluoro-pheny...)Show SMILES Fc1ccccc1C1=N[C@@H](NC(=O)c2cc3ccccc3[nH]2)C(=O)N2CCc3cccc1c23 |t:8| Show InChI InChI=1S/C26H19FN4O2/c27-19-10-3-2-8-17(19)22-18-9-5-7-15-12-13-31(23(15)18)26(33)24(29-22)30-25(32)21-14-16-6-1-4-11-20(16)28-21/h1-11,14,24,28H,12-13H2,(H,30,32)/t24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 268: 571-5 (1994)
BindingDB Entry DOI: 10.7270/Q2WQ029S |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Binding affinity towards Cholecystokinin type B receptor in rat cortex synaptosomes using [125I]BH-CCK-8 as radioligand |
J Med Chem 43: 2350-5 (2000)
BindingDB Entry DOI: 10.7270/Q2FX7B4J |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester
Curated by ChEMBL
| Assay Description
Binding affinity for Cholecystokinin type B receptor using [125I]-BH-CCK-8 in rat cortex synaptosomes |
J Med Chem 40: 4302-7 (1998)
Article DOI: 10.1021/jm970477u
BindingDB Entry DOI: 10.7270/Q2B858TF |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50005463

((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)c2cc3ccccc3[nH]2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C25H20N4O2/c1-29-21-14-8-6-12-18(21)22(16-9-3-2-4-10-16)27-23(25(29)31)28-24(30)20-15-17-11-5-7-13-19(17)26-20/h2-15,23,26H,1H3,(H,28,30)/t23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Occupational and Environmental Health
Curated by PDSP Ki Database
| |
Br J Pharmacol 117: 1558-64 (1996)
Article DOI: 10.1111/j.1476-5381.1996.tb15321.x
BindingDB Entry DOI: 10.7270/Q28W3BT3 |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1015-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QV3K1C |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM82403
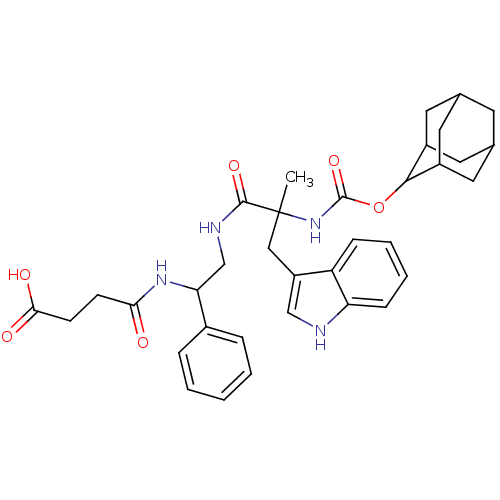
(CAS_108186 | CI-988 | NSC_108186)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NCC(NC(=O)CCC(O)=O)c1ccccc1 |TLB:25:24:18.19.20:22,THB:15:16:18.19.20:22,20:19:16:21.22.23,20:21:16:18.19.25,25:19:22:16.23.24,(.76,2.29,;.32,.82,;-.11,-.66,;.95,-1.78,;.68,-3.29,;2.03,-4.02,;3.15,-2.96,;4.68,-3.07,;5.55,-1.8,;4.88,-.42,;3.35,-.3,;2.48,-1.57,;1.8,.38,;2.92,1.45,;2.55,2.94,;4.39,1.01,;5.51,2.08,;6.88,2.77,;6.88,4.17,;7.72,5.64,;9.09,4.95,;9.09,3.55,;8.25,2.08,;7.72,2.85,;6.35,3.55,;6.35,4.95,;-1.15,1.25,;-1.52,2.75,;-2.27,.19,;-3.75,.62,;-4.86,-.44,;-6.34,-.01,;-7.45,-1.07,;-7.09,-2.57,;-8.93,-.64,;-10.04,-1.7,;-11.52,-1.27,;-11.89,.23,;-12.64,-2.33,;-4.5,-1.94,;-3.02,-2.37,;-2.65,-3.87,;-3.77,-4.93,;-5.25,-4.5,;-5.61,-3,)| Show InChI InChI=1S/C35H42N4O6/c1-35(18-26-19-36-28-10-6-5-9-27(26)28,39-34(44)45-32-24-14-21-13-22(16-24)17-25(32)15-21)33(43)37-20-29(23-7-3-2-4-8-23)38-30(40)11-12-31(41)42/h2-10,19,21-22,24-25,29,32,36H,11-18,20H2,1H3,(H,37,43)(H,38,40)(H,39,44)(H,41,42) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1015-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QV3K1C |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM82404
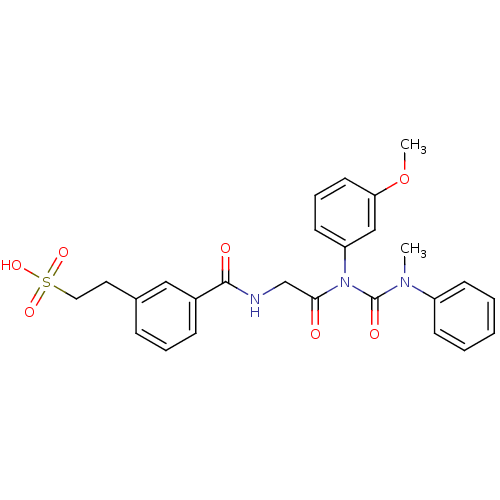
(Glycinamide,N-[[[3-(2-sulfoethyl)phenyl]amino]carb...)Show SMILES COc1cccc(c1)N(C(=O)CNC(=O)c1cccc(CCS(O)(=O)=O)c1)C(=O)N(C)c1ccccc1 Show InChI InChI=1S/C26H27N3O7S/c1-28(21-10-4-3-5-11-21)26(32)29(22-12-7-13-23(17-22)36-2)24(30)18-27-25(31)20-9-6-8-19(16-20)14-15-37(33,34)35/h3-13,16-17H,14-15,18H2,1-2H3,(H,27,31)(H,33,34,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1015-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QV3K1C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50005463

((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)c2cc3ccccc3[nH]2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C25H20N4O2/c1-29-21-14-8-6-12-18(21)22(16-9-3-2-4-10-16)27-23(25(29)31)28-24(30)20-15-17-11-5-7-13-19(17)26-20/h2-15,23,26H,1H3,(H,28,30)/t23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 269: 725-31 (1994)
BindingDB Entry DOI: 10.7270/Q2WD3Z2F |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 124: 57-73 (1996)
Article DOI: 10.1007/bf02245606
BindingDB Entry DOI: 10.7270/Q2610XV6 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM82304

(CAS_122077 | NSC_122077 | SR 27897)Show SMILES OC(=O)Cn1c(cc2ccccc12)C(=O)Nc1nc(cs1)-c1ccccc1Cl Show InChI InChI=1S/C20H14ClN3O3S/c21-14-7-3-2-6-13(14)15-11-28-20(22-15)23-19(27)17-9-12-5-1-4-8-16(12)24(17)10-18(25)26/h1-9,11H,10H2,(H,25,26)(H,22,23,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
Eur J Pharmacol 232: 13-9 (1993)
Article DOI: 10.1016/0014-2999(93)90722-t
BindingDB Entry DOI: 10.7270/Q2P26WN2 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Affinity to inhibit [3H]pCCK-8 specific binding on rat brain Cholecystokinin type B receptor expressed in CHO cells |
J Med Chem 40: 647-58 (1997)
Article DOI: 10.1021/jm9603072
BindingDB Entry DOI: 10.7270/Q2PG1SDX |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against Cholecystokinin type B receptor expressed in CHO cells on the rat brain. |
J Med Chem 43: 3614-23 (2000)
BindingDB Entry DOI: 10.7270/Q2RN373C |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM81963

(CAS_9011-97-6 | CCK | CCK33 | CHOLECYSTOKININ)Show SMILES CCC(C)C(NC(=O)C(CCCNC(N)=N)NC(=O)C(Cc1c[nH]cn1)NC(=O)C(CO)NC(=O)C1CCCN1C(=O)C(CC(O)=O)NC(=O)C(CC(C)C)NC(=O)C(CC(N)=O)NC(=O)C(CCC(N)=O)NC(=O)C(CC(C)C)NC(=O)C(CC(N)=O)NC(=O)C(CCCCN)NC(=O)C(NC(=O)C(NC(=O)C(CO)NC(=O)C(CCSC)NC(=O)C(CCCNC(N)=N)NC(=O)CNC(=O)C(CO)NC(=O)C1CCCN1C(=O)C(C)NC(=O)C(N)CCCCN)C(C)CC)C(C)C)C(=O)NC(CO)C(=O)NC(CC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NC(CC(O)=O)C(=O)NC(C(=O)NC(CCSC)C(=O)NCC(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCSC)C(=O)NC(CC(O)=O)C(=O)NC(Cc1ccccc1)C(N)=O)c1ccc(OS(O)(=O)=O)cc1 Show InChI InChI=1S/C166H261N51O52S4/c1-15-84(9)130(159(261)211-116(78-220)154(256)206-110(68-125(227)228)150(252)191-97(38-27-54-182-165(176)177)139(241)205-112(70-127(231)232)152(254)215-132(88-42-44-91(45-43-88)269-273(266,267)268)161(263)197-100(48-58-270-12)135(237)185-75-124(226)190-106(64-89-72-184-94-35-21-20-33-92(89)94)146(248)195-101(49-59-271-13)141(243)204-111(69-126(229)230)151(253)198-103(133(173)235)63-87-31-18-17-19-32-87)213-143(245)98(39-28-55-183-166(178)179)192-147(249)107(65-90-73-180-80-187-90)201-153(255)115(77-219)210-157(259)119-41-30-57-217(119)163(265)113(71-128(233)234)207-145(247)105(62-82(5)6)200-149(251)109(67-122(172)224)203-140(242)99(46-47-120(170)222)193-144(246)104(61-81(3)4)199-148(250)108(66-121(171)223)202-138(240)96(36-23-25-52-168)196-158(260)129(83(7)8)212-160(262)131(85(10)16-2)214-155(257)117(79-221)208-142(244)102(50-60-272-14)194-137(239)95(37-26-53-181-164(174)175)189-123(225)74-186-136(238)114(76-218)209-156(258)118-40-29-56-216(118)162(264)86(11)188-134(236)93(169)34-22-24-51-167/h17-21,31-33,35,42-45,72-73,80-86,93,95-119,129-132,184,218-221H,15-16,22-30,34,36-41,46-71,74-79,167-169H2,1-14H3,(H2,170,222)(H2,171,223)(H2,172,224)(H2,173,235)(H,180,187)(H,185,237)(H,186,238)(H,188,236)(H,189,225)(H,190,226)(H,191,252)(H,192,249)(H,193,246)(H,194,239)(H,195,248)(H,196,260)(H,197,263)(H,198,253)(H,199,250)(H,200,251)(H,201,255)(H,202,240)(H,203,242)(H,204,243)(H,205,241)(H,206,256)(H,207,247)(H,208,244)(H,209,258)(H,210,259)(H,211,261)(H,212,262)(H,213,245)(H,214,257)(H,215,254)(H,227,228)(H,229,230)(H,231,232)(H,233,234)(H4,174,175,181)(H4,176,177,182)(H4,178,179,183)(H,266,267,268) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche
Curated by PDSP Ki Database
| |
Eur J Pharmacol 232: 13-9 (1993)
Article DOI: 10.1016/0014-2999(93)90722-t
BindingDB Entry DOI: 10.7270/Q2P26WN2 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Ability to displace 1 nM [3H]pCCK-8 from rat Cholecystokinin type B receptor stably expressing in CHO cells |
Bioorg Med Chem Lett 14: 369-72 (2003)
BindingDB Entry DOI: 10.7270/Q2RJ4K1Z |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50092405
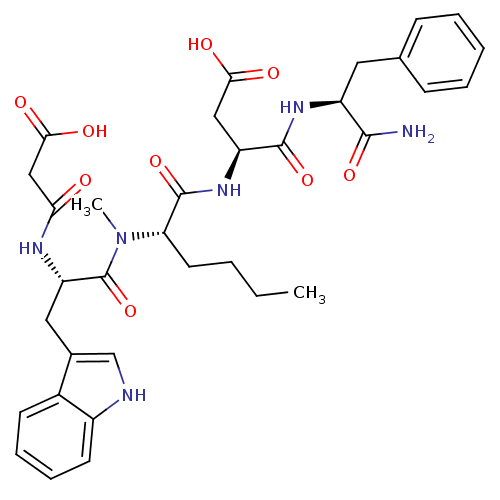
((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-((S)-2-{[...)Show SMILES CCCC[C@H](N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C34H42N6O9/c1-3-4-14-27(33(48)39-25(17-29(42)43)32(47)38-24(31(35)46)15-20-10-6-5-7-11-20)40(2)34(49)26(37-28(41)18-30(44)45)16-21-19-36-23-13-9-8-12-22(21)23/h5-13,19,24-27,36H,3-4,14-18H2,1-2H3,(H2,35,46)(H,37,41)(H,38,47)(H,39,48)(H,42,43)(H,44,45)/t24-,25-,26-,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against Cholecystokinin type B receptor expressed in CHO cells on the rat brain. |
J Med Chem 43: 3614-23 (2000)
BindingDB Entry DOI: 10.7270/Q2RN373C |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM82404

(Glycinamide,N-[[[3-(2-sulfoethyl)phenyl]amino]carb...)Show SMILES COc1cccc(c1)N(C(=O)CNC(=O)c1cccc(CCS(O)(=O)=O)c1)C(=O)N(C)c1ccccc1 Show InChI InChI=1S/C26H27N3O7S/c1-28(21-10-4-3-5-11-21)26(32)29(22-12-7-13-23(17-22)36-2)24(30)18-27-25(31)20-9-6-8-19(16-20)14-15-37(33,34)35/h3-13,16-17H,14-15,18H2,1-2H3,(H,27,31)(H,33,34,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1015-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QV3K1C |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM81963

(CAS_9011-97-6 | CCK | CCK33 | CHOLECYSTOKININ)Show SMILES CCC(C)C(NC(=O)C(CCCNC(N)=N)NC(=O)C(Cc1c[nH]cn1)NC(=O)C(CO)NC(=O)C1CCCN1C(=O)C(CC(O)=O)NC(=O)C(CC(C)C)NC(=O)C(CC(N)=O)NC(=O)C(CCC(N)=O)NC(=O)C(CC(C)C)NC(=O)C(CC(N)=O)NC(=O)C(CCCCN)NC(=O)C(NC(=O)C(NC(=O)C(CO)NC(=O)C(CCSC)NC(=O)C(CCCNC(N)=N)NC(=O)CNC(=O)C(CO)NC(=O)C1CCCN1C(=O)C(C)NC(=O)C(N)CCCCN)C(C)CC)C(C)C)C(=O)NC(CO)C(=O)NC(CC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NC(CC(O)=O)C(=O)NC(C(=O)NC(CCSC)C(=O)NCC(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCSC)C(=O)NC(CC(O)=O)C(=O)NC(Cc1ccccc1)C(N)=O)c1ccc(OS(O)(=O)=O)cc1 Show InChI InChI=1S/C166H261N51O52S4/c1-15-84(9)130(159(261)211-116(78-220)154(256)206-110(68-125(227)228)150(252)191-97(38-27-54-182-165(176)177)139(241)205-112(70-127(231)232)152(254)215-132(88-42-44-91(45-43-88)269-273(266,267)268)161(263)197-100(48-58-270-12)135(237)185-75-124(226)190-106(64-89-72-184-94-35-21-20-33-92(89)94)146(248)195-101(49-59-271-13)141(243)204-111(69-126(229)230)151(253)198-103(133(173)235)63-87-31-18-17-19-32-87)213-143(245)98(39-28-55-183-166(178)179)192-147(249)107(65-90-73-180-80-187-90)201-153(255)115(77-219)210-157(259)119-41-30-57-217(119)163(265)113(71-128(233)234)207-145(247)105(62-82(5)6)200-149(251)109(67-122(172)224)203-140(242)99(46-47-120(170)222)193-144(246)104(61-81(3)4)199-148(250)108(66-121(171)223)202-138(240)96(36-23-25-52-168)196-158(260)129(83(7)8)212-160(262)131(85(10)16-2)214-155(257)117(79-221)208-142(244)102(50-60-272-14)194-137(239)95(37-26-53-181-164(174)175)189-123(225)74-186-136(238)114(76-218)209-156(258)118-40-29-56-216(118)162(264)86(11)188-134(236)93(169)34-22-24-51-167/h17-21,31-33,35,42-45,72-73,80-86,93,95-119,129-132,184,218-221H,15-16,22-30,34,36-41,46-71,74-79,167-169H2,1-14H3,(H2,170,222)(H2,171,223)(H2,172,224)(H2,173,235)(H,180,187)(H,185,237)(H,186,238)(H,188,236)(H,189,225)(H,190,226)(H,191,252)(H,192,249)(H,193,246)(H,194,239)(H,195,248)(H,196,260)(H,197,263)(H,198,253)(H,199,250)(H,200,251)(H,201,255)(H,202,240)(H,203,242)(H,204,243)(H,205,241)(H,206,256)(H,207,247)(H,208,244)(H,209,258)(H,210,259)(H,211,261)(H,212,262)(H,213,245)(H,214,257)(H,215,254)(H,227,228)(H,229,230)(H,231,232)(H,233,234)(H4,174,175,181)(H4,176,177,182)(H4,178,179,183)(H,266,267,268) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 162: 273-80 (1989)
Article DOI: 10.1016/0014-2999(89)90290-2
BindingDB Entry DOI: 10.7270/Q2QF8RB7 |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM50061220

(1-((S)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)Nc2cccc(C)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-9-8-12-18(15-16)25-24(30)27-22-23(29)28(2)20-14-7-6-13-19(20)21(26-22)17-10-4-3-5-11-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 162: 273-80 (1989)
Article DOI: 10.1016/0014-2999(89)90290-2
BindingDB Entry DOI: 10.7270/Q2QF8RB7 |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1015-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QV3K1C |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM82514
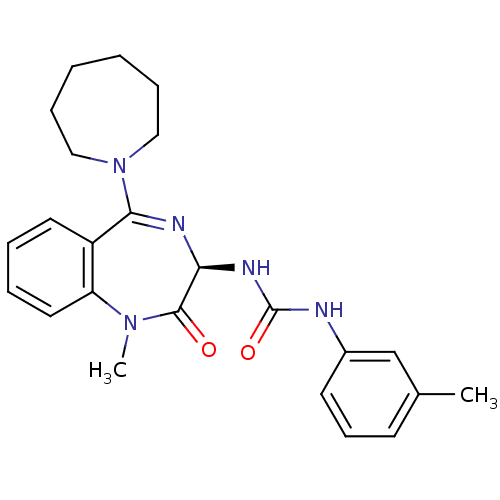
(1-[[(3R)-2,3-Dihydro-1-methyl-2-oxo-5-[(hexahydro-...)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(C)c2)C1=O)N1CCCCCC1 |c:9| Show InChI InChI=1S/C24H29N5O2/c1-17-10-9-11-18(16-17)25-24(31)27-21-23(30)28(2)20-13-6-5-12-19(20)22(26-21)29-14-7-3-4-8-15-29/h5-6,9-13,16,21H,3-4,7-8,14-15H2,1-2H3,(H2,25,27,31)/t21-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Mol Pharmacol 46: 943-8 (1994)
BindingDB Entry DOI: 10.7270/Q2QR4VMN |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM84958

(2-[[(R)-2-(1H-Indol-2-ylcarbonylamino)-3-(4-benzhy...)Show SMILES OC(=O)c1cccnc1SC[C@H](NC(=O)c1cc2ccccc2[nH]1)C(=O)N1CCN(CC1)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C35H33N5O4S/c41-32(29-22-26-14-7-8-16-28(26)37-29)38-30(23-45-33-27(35(43)44)15-9-17-36-33)34(42)40-20-18-39(19-21-40)31(24-10-3-1-4-11-24)25-12-5-2-6-13-25/h1-17,22,30-31,37H,18-21,23H2,(H,38,41)(H,43,44)/t30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Occupational and Environmental Health
Curated by PDSP Ki Database
| |
Br J Pharmacol 117: 1558-64 (1996)
Article DOI: 10.1111/j.1476-5381.1996.tb15321.x
BindingDB Entry DOI: 10.7270/Q28W3BT3 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50061832
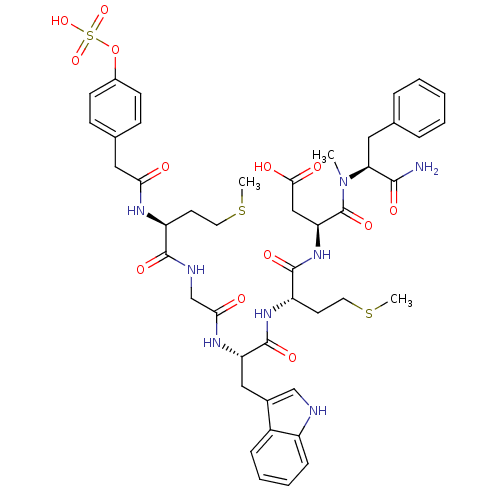
((S)-N-((S)-1-Carbamoyl-2-phenyl-ethyl)-3-{(S)-2-[(...)Show SMILES CSCC[C@H](NC(=O)Cc1ccc(OS(O)(=O)=O)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N(C)[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C45H56N8O13S3/c1-53(37(41(46)58)21-27-9-5-4-6-10-27)45(62)36(24-40(56)57)52-43(60)34(18-20-68-3)51-44(61)35(23-29-25-47-32-12-8-7-11-31(29)32)50-39(55)26-48-42(59)33(17-19-67-2)49-38(54)22-28-13-15-30(16-14-28)66-69(63,64)65/h4-16,25,33-37,47H,17-24,26H2,1-3H3,(H2,46,58)(H,48,59)(H,49,54)(H,50,55)(H,51,61)(H,52,60)(H,56,57)(H,63,64,65)/t33-,34-,35-,36-,37-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rochester
Curated by ChEMBL
| Assay Description
Binding affinity for Cholecystokinin type B receptor using [125I]-BH-CCK-8 in rat cortex synaptosomes |
J Med Chem 40: 4302-7 (1998)
Article DOI: 10.1021/jm970477u
BindingDB Entry DOI: 10.7270/Q2B858TF |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 124: 57-73 (1996)
Article DOI: 10.1007/bf02245606
BindingDB Entry DOI: 10.7270/Q2610XV6 |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM50061220

(1-((S)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)Nc2cccc(C)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-9-8-12-18(15-16)25-24(30)27-22-23(29)28(2)20-14-7-6-13-19(20)21(26-22)17-10-4-3-5-11-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 162: 273-80 (1989)
Article DOI: 10.1016/0014-2999(89)90290-2
BindingDB Entry DOI: 10.7270/Q2QF8RB7 |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM82403

(CAS_108186 | CI-988 | NSC_108186)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)NCC(NC(=O)CCC(O)=O)c1ccccc1 |TLB:25:24:18.19.20:22,THB:15:16:18.19.20:22,20:19:16:21.22.23,20:21:16:18.19.25,25:19:22:16.23.24,(.76,2.29,;.32,.82,;-.11,-.66,;.95,-1.78,;.68,-3.29,;2.03,-4.02,;3.15,-2.96,;4.68,-3.07,;5.55,-1.8,;4.88,-.42,;3.35,-.3,;2.48,-1.57,;1.8,.38,;2.92,1.45,;2.55,2.94,;4.39,1.01,;5.51,2.08,;6.88,2.77,;6.88,4.17,;7.72,5.64,;9.09,4.95,;9.09,3.55,;8.25,2.08,;7.72,2.85,;6.35,3.55,;6.35,4.95,;-1.15,1.25,;-1.52,2.75,;-2.27,.19,;-3.75,.62,;-4.86,-.44,;-6.34,-.01,;-7.45,-1.07,;-7.09,-2.57,;-8.93,-.64,;-10.04,-1.7,;-11.52,-1.27,;-11.89,.23,;-12.64,-2.33,;-4.5,-1.94,;-3.02,-2.37,;-2.65,-3.87,;-3.77,-4.93,;-5.25,-4.5,;-5.61,-3,)| Show InChI InChI=1S/C35H42N4O6/c1-35(18-26-19-36-28-10-6-5-9-27(26)28,39-34(44)45-32-24-14-21-13-22(16-24)17-25(32)15-21)33(43)37-20-29(23-7-3-2-4-8-23)38-30(40)11-12-31(41)42/h2-10,19,21-22,24-25,29,32,36H,11-18,20H2,1H3,(H,37,43)(H,38,40)(H,39,44)(H,41,42) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1015-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QV3K1C |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM50061220

(1-((S)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)Nc2cccc(C)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-9-8-12-18(15-16)25-24(30)27-22-23(29)28(2)20-14-7-6-13-19(20)21(26-22)17-10-4-3-5-11-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 162: 273-80 (1989)
Article DOI: 10.1016/0014-2999(89)90290-2
BindingDB Entry DOI: 10.7270/Q2QF8RB7 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Dog) | BDBM50411344
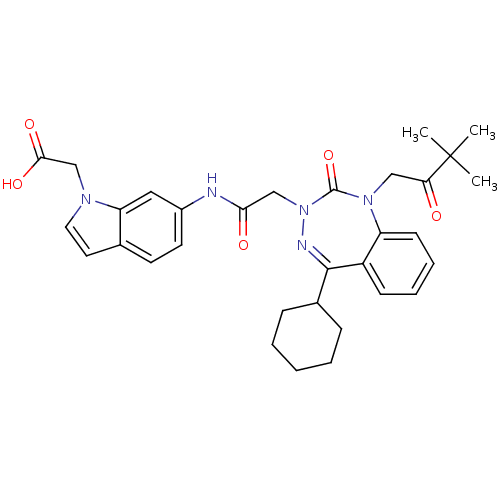
(CHEMBL389711)Show SMILES CC(C)(C)C(=O)CN1c2ccccc2C(=NN(CC(=O)Nc2ccc3ccn(CC(O)=O)c3c2)C1=O)C1CCCCC1 |c:15| Show InChI InChI=1S/C32H37N5O5/c1-32(2,3)27(38)18-36-25-12-8-7-11-24(25)30(22-9-5-4-6-10-22)34-37(31(36)42)19-28(39)33-23-14-13-21-15-16-35(20-29(40)41)26(21)17-23/h7-8,11-17,22H,4-6,9-10,18-20H2,1-3H3,(H,33,39)(H,40,41) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [125I]BH-CCK8-8S from CCK2 receptor in canine gastric mucosa |
J Med Chem 50: 3101-12 (2007)
Checked by Author
Article DOI: 10.1021/jm070139l
BindingDB Entry DOI: 10.7270/Q2QJ7JHN |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM82235
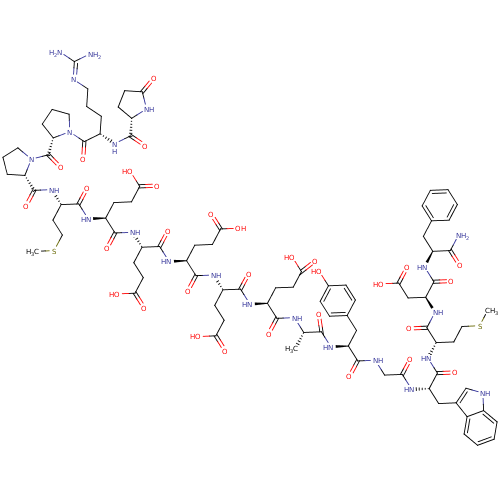
(Gastrin I | Gastrin-17 | Gastrin-I-(1-17))Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H]1CCC(=O)N1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,wU:4.4,138.143,38.41,52.60,70.78,88.95,96.98,wD:8.20,130.135,26.37,43.51,61.69,79.87,103.106,110.122,121.125,(26,6.14,;24.67,5.35,;24.67,3.8,;23.32,3.01,;23.32,1.49,;22,.73,;20.68,1.49,;20.68,3.04,;19.35,.73,;19.35,-.76,;20.68,-1.52,;21.94,-.7,;23.15,-1.69,;22.59,-3.1,;23.29,-4.48,;22.45,-5.77,;20.9,-5.71,;20.2,-4.31,;21.04,-2.98,;18,1.49,;16.68,.7,;16.68,-.76,;15.36,1.49,;14.03,.7,;12.68,1.46,;12.68,3.01,;11.36,.68,;11.36,-.79,;12.71,-1.55,;12.71,-3.1,;14.03,-3.86,;15.36,-3.07,;16.71,-3.86,;15.36,-1.55,;14.03,-.79,;10.01,1.44,;8.66,.68,;8.66,-.82,;7.33,1.44,;7.3,2.98,;6.01,.65,;4.66,1.44,;4.66,2.98,;3.34,.65,;3.34,-.84,;4.66,-1.6,;4.66,-3.15,;6.01,-3.91,;3.34,-3.91,;2.01,1.44,;.66,.65,;.66,-.84,;-.61,1.44,;-.61,2.96,;.66,3.74,;.66,5.32,;2.01,6.11,;-.61,6.11,;-1.93,.65,;-3.28,1.41,;-3.28,2.96,;-4.6,.65,;-4.6,-.84,;-3.28,-1.6,;-3.28,-3.15,;-1.93,-3.94,;-4.6,-3.94,;-5.93,1.41,;-7.28,.65,;-7.28,-.84,;-8.6,1.44,;-8.6,2.96,;-7.28,3.74,;-7.28,5.32,;-5.93,6.11,;-8.6,6.11,;-9.95,.65,;-11.33,1.44,;-11.33,2.98,;-12.65,.65,;-12.65,-.82,;-11.33,-1.6,;-11.33,-3.15,;-9.95,-3.94,;-12.65,-3.91,;-13.98,1.44,;-15.33,.68,;-15.33,-.79,;-16.65,1.44,;-16.65,2.96,;-15.3,3.77,;-15.3,5.29,;-13.98,6.08,;-17.97,.68,;-19.32,1.44,;-19.32,2.98,;-20.62,.68,;-19.69,1.83,;-20.39,3.26,;-21.91,2.96,;-21.91,1.44,;-23.24,.68,;-23.24,-.96,;-24.7,1.52,;-23.97,.34,;-24.56,-.98,;-26.16,-.7,;-26.16,.68,;-27.52,1.44,;-27.52,2.98,;-28.84,.68,;-28.84,-.82,;-27.52,-1.55,;-27.52,-3.1,;-26.14,-3.86,;-26.11,-5.4,;-27.52,-6.16,;-24.78,-6.16,;-30.19,1.44,;-31.51,.68,;-31.51,-.82,;-32.84,1.41,;-31.85,.28,;-32.55,-1.1,;-34.07,-.79,;-35.37,-1.58,;-34.07,.68,;24.67,.73,;24.67,-.73,;26,1.49,;27.35,.7,;27.35,-.76,;28.7,-1.55,;30.02,-.76,;28.7,-3.07,;28.7,1.49,;28.7,3.04,;30.02,.7,;31.34,1.49,;31.34,3.01,;32.7,3.83,;32.7,5.38,;34.02,6.16,;35.37,5.38,;35.37,3.83,;34.02,3.01,;32.7,.7,;34.02,1.49,;32.7,-.76,)| Show InChI InChI=1S/C94H128N22O31S2/c1-48(79(133)113-65(43-50-19-21-52(117)22-20-50)80(134)100-47-71(119)103-66(44-51-46-99-54-15-8-7-14-53(51)54)89(143)109-61(35-40-148-2)88(142)114-67(45-77(130)131)90(144)112-64(78(95)132)42-49-12-5-4-6-13-49)101-81(135)56(24-30-72(120)121)104-83(137)57(25-31-73(122)123)105-84(138)58(26-32-74(124)125)106-85(139)59(27-33-75(126)127)107-86(140)60(28-34-76(128)129)108-87(141)62(36-41-149-3)110-91(145)68-17-10-38-115(68)93(147)69-18-11-39-116(69)92(146)63(16-9-37-98-94(96)97)111-82(136)55-23-29-70(118)102-55/h4-8,12-15,19-22,46,48,55-69,99,117H,9-11,16-18,23-45,47H2,1-3H3,(H2,95,132)(H,100,134)(H,101,135)(H,102,118)(H,103,119)(H,104,137)(H,105,138)(H,106,139)(H,107,140)(H,108,141)(H,109,143)(H,110,145)(H,111,136)(H,112,144)(H,113,133)(H,114,142)(H,120,121)(H,122,123)(H,124,125)(H,126,127)(H,128,129)(H,130,131)(H4,96,97,98)/t48-,55-,56-,57-,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 269: 725-31 (1994)
BindingDB Entry DOI: 10.7270/Q2WD3Z2F |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
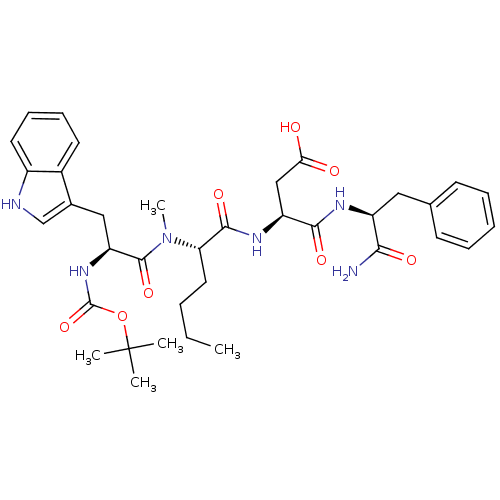
(RAT) | BDBM50092398
((S)-3-((S)-2-{[(S)-2-tert-Butoxycarbonylamino-3-(1...)Show SMILES CCCC[C@H](N(C)C(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C36H48N6O8/c1-6-7-17-29(33(47)40-27(20-30(43)44)32(46)39-26(31(37)45)18-22-13-9-8-10-14-22)42(5)34(48)28(41-35(49)50-36(2,3)4)19-23-21-38-25-16-12-11-15-24(23)25/h8-16,21,26-29,38H,6-7,17-20H2,1-5H3,(H2,37,45)(H,39,46)(H,40,47)(H,41,49)(H,43,44)/t26-,27-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against Cholecystokinin type B receptor expressed in CHO cells on the rat brain. |
J Med Chem 43: 3614-23 (2000)
BindingDB Entry DOI: 10.7270/Q2RN373C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of [3H]- pCCK-8 binding to Cholecystokinin type B receptor of rat cerebral cortex membranes |
J Med Chem 40: 3402-7 (1997)
Article DOI: 10.1021/jm9703247
BindingDB Entry DOI: 10.7270/Q2B56HV4 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
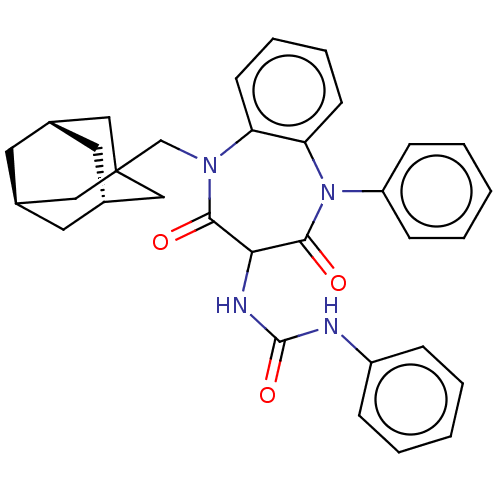
(RAT) | BDBM50472854
(CHEMBL330977)Show SMILES [H][C@]12C[C@]3([H])C[C@]([H])(C1)CC(CN1c4ccccc4N(c4ccccc4)C(=O)C(NC(=O)Nc4ccccc4)C1=O)(C2)C3 |TLB:8:1:42:6.9.5,8:6:42:1.41.2,THB:2:3:9:1.41.8,2:1:9:3.42.5| Show InChI InChI=1S/C33H34N4O3/c38-30-29(35-32(40)34-25-9-3-1-4-10-25)31(39)37(26-11-5-2-6-12-26)28-14-8-7-13-27(28)36(30)21-33-18-22-15-23(19-33)17-24(16-22)20-33/h1-14,22-24,29H,15-21H2,(H2,34,35,40)/t22-,23+,24-,29?,33? | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre
Curated by ChEMBL
| Assay Description
Inhibition by displacing [3H]CCK-8S against Cholecystokinin type B receptor of rat pancreatic membranes |
J Med Chem 43: 3596-613 (2000)
Article DOI: 10.1021/jm990967h
BindingDB Entry DOI: 10.7270/Q26H4M5V |
More data for this
Ligand-Target Pair | |
Cholecystokinin
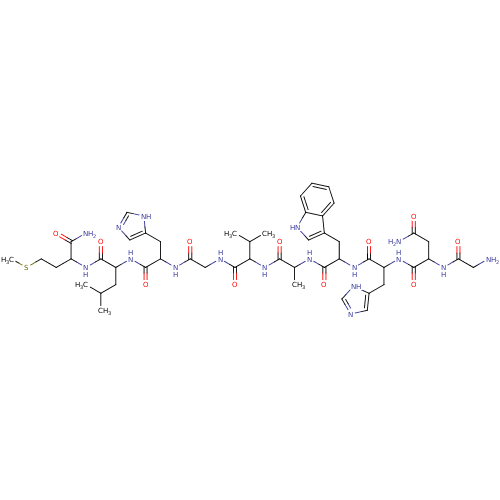
(GUINEA PIG) | BDBM81964
(CAS_5486814 | Gastrin | NSC_5486814 | Neuromedin C)Show SMILES CSCCC(NC(=O)C(CC(C)C)NC(=O)C(Cc1cnc[nH]1)NC(=O)CNC(=O)C(NC(=O)C(C)NC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)C(Cc1cnc[nH]1)NC(=O)C(CC(N)=O)NC(=O)CN)C(C)C)C(N)=O Show InChI InChI=1S/C50H73N17O11S/c1-25(2)13-34(46(74)63-33(43(53)71)11-12-79-6)64-47(75)36(15-29-20-54-23-58-29)62-41(70)22-57-50(78)42(26(3)4)67-44(72)27(5)60-45(73)35(14-28-19-56-32-10-8-7-9-31(28)32)65-48(76)37(16-30-21-55-24-59-30)66-49(77)38(17-39(52)68)61-40(69)18-51/h7-10,19-21,23-27,33-38,42,56H,11-18,22,51H2,1-6H3,(H2,52,68)(H2,53,71)(H,54,58)(H,55,59)(H,57,78)(H,60,73)(H,61,69)(H,62,70)(H,63,74)(H,64,75)(H,65,76)(H,66,77)(H,67,72) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 162: 273-80 (1989)
Article DOI: 10.1016/0014-2999(89)90290-2
BindingDB Entry DOI: 10.7270/Q2QF8RB7 |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM81962
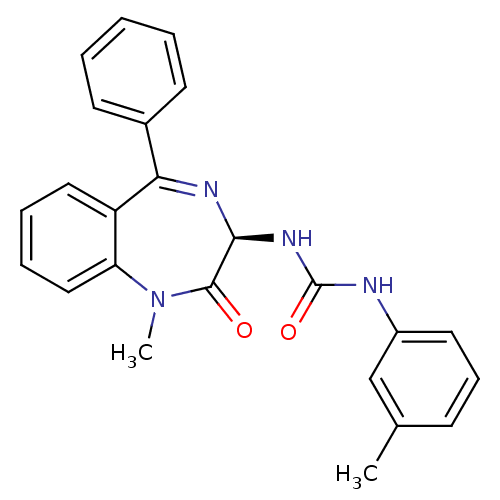
(S-L-365,260)Show SMILES CN1c2ccccc2C(=N[C@@H](NC(=O)Nc2cccc(C)c2)C1=O)c1ccccc1 |r,c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-9-8-12-18(15-16)25-24(30)27-22-23(29)28(2)20-14-7-6-13-19(20)21(26-22)17-10-4-3-5-11-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 162: 273-80 (1989)
Article DOI: 10.1016/0014-2999(89)90290-2
BindingDB Entry DOI: 10.7270/Q2QF8RB7 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Dog) | BDBM50411348
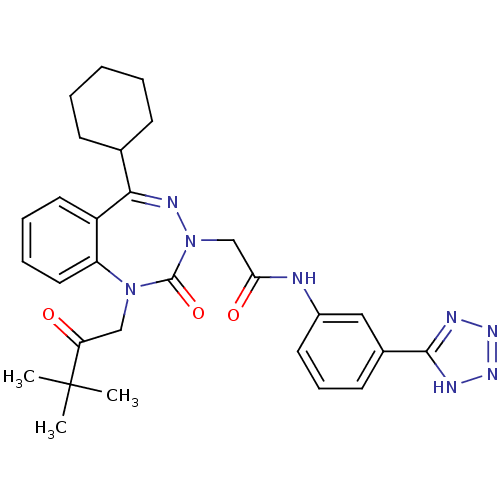
(CHEMBL226533)Show SMILES CC(C)(C)C(=O)CN1c2ccccc2C(=NN(CC(=O)Nc2cccc(c2)-c2nnn[nH]2)C1=O)C1CCCCC1 |c:15| Show InChI InChI=1S/C29H34N8O3/c1-29(2,3)24(38)17-36-23-15-8-7-14-22(23)26(19-10-5-4-6-11-19)33-37(28(36)40)18-25(39)30-21-13-9-12-20(16-21)27-31-34-35-32-27/h7-9,12-16,19H,4-6,10-11,17-18H2,1-3H3,(H,30,39)(H,31,32,34,35) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [125I]BH-CCK8-8S from CCK2 receptor in canine gastric mucosa |
J Med Chem 50: 3101-12 (2007)
Checked by Author
Article DOI: 10.1021/jm070139l
BindingDB Entry DOI: 10.7270/Q2QJ7JHN |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM82241
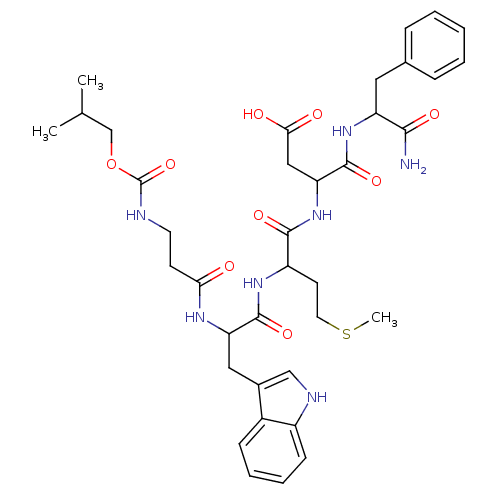
(CAS_5534-95-2 | NSC_444007 | Pentagastrin)Show SMILES CSCCC(NC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)CCNC(=O)OCC(C)C)C(=O)NC(CC(O)=O)C(=O)NC(Cc1ccccc1)C(N)=O Show InChI InChI=1S/C37H49N7O9S/c1-22(2)21-53-37(52)39-15-13-31(45)41-29(18-24-20-40-26-12-8-7-11-25(24)26)35(50)42-27(14-16-54-3)34(49)44-30(19-32(46)47)36(51)43-28(33(38)48)17-23-9-5-4-6-10-23/h4-12,20,22,27-30,40H,13-19,21H2,1-3H3,(H2,38,48)(H,39,52)(H,41,45)(H,42,50)(H,43,51)(H,44,49)(H,46,47) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 273: 1015-22 (1995)
BindingDB Entry DOI: 10.7270/Q2QV3K1C |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM50061220

(1-((S)-1-Methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benz...)Show SMILES CN1c2ccccc2C(=N[C@H](NC(=O)Nc2cccc(C)c2)C1=O)c1ccccc1 |c:9| Show InChI InChI=1S/C24H22N4O2/c1-16-9-8-12-18(15-16)25-24(30)27-22-23(29)28(2)20-14-7-6-13-19(20)21(26-22)17-10-4-3-5-11-17/h3-15,22H,1-2H3,(H2,25,27,30)/t22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 162: 273-80 (1989)
Article DOI: 10.1016/0014-2999(89)90290-2
BindingDB Entry DOI: 10.7270/Q2QF8RB7 |
More data for this
Ligand-Target Pair | |
Cholecystokinin
(GUINEA PIG) | BDBM81964

(CAS_5486814 | Gastrin | NSC_5486814 | Neuromedin C)Show SMILES CSCCC(NC(=O)C(CC(C)C)NC(=O)C(Cc1cnc[nH]1)NC(=O)CNC(=O)C(NC(=O)C(C)NC(=O)C(Cc1c[nH]c2ccccc12)NC(=O)C(Cc1cnc[nH]1)NC(=O)C(CC(N)=O)NC(=O)CN)C(C)C)C(N)=O Show InChI InChI=1S/C50H73N17O11S/c1-25(2)13-34(46(74)63-33(43(53)71)11-12-79-6)64-47(75)36(15-29-20-54-23-58-29)62-41(70)22-57-50(78)42(26(3)4)67-44(72)27(5)60-45(73)35(14-28-19-56-32-10-8-7-9-31(28)32)65-48(76)37(16-30-21-55-24-59-30)66-49(77)38(17-39(52)68)61-40(69)18-51/h7-10,19-21,23-27,33-38,42,56H,11-18,22,51H2,1-6H3,(H2,52,68)(H2,53,71)(H,54,58)(H,55,59)(H,57,78)(H,60,73)(H,61,69)(H,62,70)(H,63,74)(H,64,75)(H,65,76)(H,66,77)(H,67,72) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 162: 273-80 (1989)
Article DOI: 10.1016/0014-2999(89)90290-2
BindingDB Entry DOI: 10.7270/Q2QF8RB7 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Dog) | BDBM50411336

(CHEMBL227330)Show SMILES OC(=O)CCc1cccc(NC(=O)CN2N=C(C3CCCCC3)c3ccccc3N(CC(=O)C3CCCC3)C2=O)c1 |t:15| Show InChI InChI=1S/C32H38N4O5/c37-28(23-10-4-5-11-23)20-35-27-16-7-6-15-26(27)31(24-12-2-1-3-13-24)34-36(32(35)41)21-29(38)33-25-14-8-9-22(19-25)17-18-30(39)40/h6-9,14-16,19,23-24H,1-5,10-13,17-18,20-21H2,(H,33,38)(H,39,40) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [125I]BH-CCK8-8S from CCK2 receptor in canine gastric mucosa |
J Med Chem 50: 3101-12 (2007)
Checked by Author
Article DOI: 10.1021/jm070139l
BindingDB Entry DOI: 10.7270/Q2QJ7JHN |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Dog) | BDBM50411341
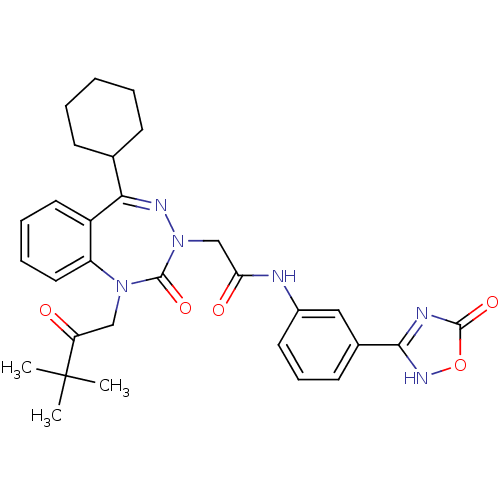
(CHEMBL226583)Show SMILES CC(C)(C)C(=O)CN1c2ccccc2C(=NN(CC(=O)Nc2cccc(c2)-c2nc(=O)o[nH]2)C1=O)C1CCCCC1 |c:15| Show InChI InChI=1S/C30H34N6O5/c1-30(2,3)24(37)17-35-23-15-8-7-14-22(23)26(19-10-5-4-6-11-19)33-36(29(35)40)18-25(38)31-21-13-9-12-20(16-21)27-32-28(39)41-34-27/h7-9,12-16,19H,4-6,10-11,17-18H2,1-3H3,(H,31,38)(H,32,34,39) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [125I]BH-CCK8-8S from CCK2 receptor in canine gastric mucosa |
J Med Chem 50: 3101-12 (2007)
Checked by Author
Article DOI: 10.1021/jm070139l
BindingDB Entry DOI: 10.7270/Q2QJ7JHN |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Dog) | BDBM50411339
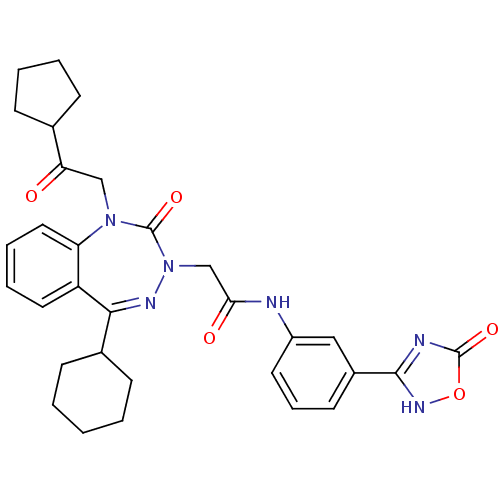
(CHEMBL227276)Show SMILES O=C(CN1N=C(C2CCCCC2)c2ccccc2N(CC(=O)C2CCCC2)C1=O)Nc1cccc(c1)-c1nc(=O)o[nH]1 |t:4| Show InChI InChI=1S/C31H34N6O5/c38-26(20-9-4-5-10-20)18-36-25-16-7-6-15-24(25)28(21-11-2-1-3-12-21)34-37(31(36)41)19-27(39)32-23-14-8-13-22(17-23)29-33-30(40)42-35-29/h6-8,13-17,20-21H,1-5,9-12,18-19H2,(H,32,39)(H,33,35,40) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [125I]BH-CCK8-8S from CCK2 receptor in canine gastric mucosa |
J Med Chem 50: 3101-12 (2007)
Checked by Author
Article DOI: 10.1021/jm070139l
BindingDB Entry DOI: 10.7270/Q2QJ7JHN |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
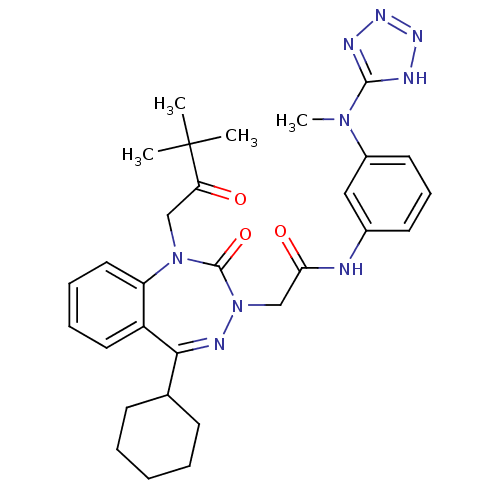
(Dog) | BDBM50411338
(CHEMBL227254)Show SMILES CN(c1nnn[nH]1)c1cccc(NC(=O)CN2N=C(C3CCCCC3)c3ccccc3N(CC(=O)C(C)(C)C)C2=O)c1 |t:18| Show InChI InChI=1S/C30H37N9O3/c1-30(2,3)25(40)18-38-24-16-9-8-15-23(24)27(20-11-6-5-7-12-20)34-39(29(38)42)19-26(41)31-21-13-10-14-22(17-21)37(4)28-32-35-36-33-28/h8-10,13-17,20H,5-7,11-12,18-19H2,1-4H3,(H,31,41)(H,32,33,35,36) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
James Black Foundation
Curated by ChEMBL
| Assay Description
Displacement of [125I]BH-CCK8-8S from CCK2 receptor in canine gastric mucosa |
J Med Chem 50: 3101-12 (2007)
Checked by Author
Article DOI: 10.1021/jm070139l
BindingDB Entry DOI: 10.7270/Q2QJ7JHN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data