

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4-hydroxy-2-oxoglutarate aldolase, mitochondrial (Homo sapiens (Human)) | BDBM267947 ((3aR,5R,6S,7R,7aR)-5- ((cyclopentylamino)methyl)-2...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | -52.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Alectos Therapeutics Inc.; Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions were carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acet... | US Patent US9718854 (2017) BindingDB Entry DOI: 10.7270/Q2CJ8GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-2-oxoglutarate aldolase, mitochondrial (Homo sapiens (Human)) | BDBM267944 ((3aR,5R,6S,7R,7aR)-5- ((cyclopropylamino)methyl)-2...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.30 | -50.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Alectos Therapeutics Inc.; Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions were carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acet... | US Patent US9718854 (2017) BindingDB Entry DOI: 10.7270/Q2CJ8GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-2-oxoglutarate aldolase, mitochondrial (Homo sapiens (Human)) | BDBM267946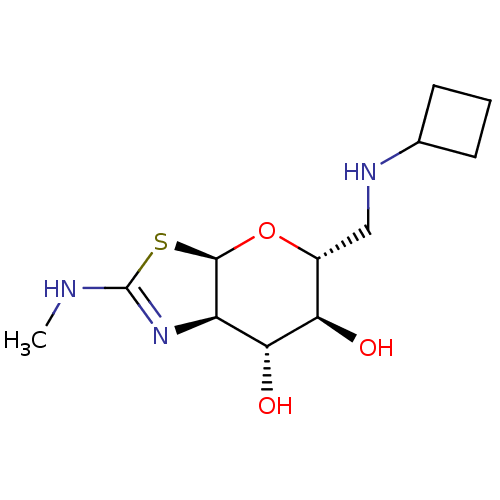 ((3aR,5R,6S,7R,7aR)-5- ((cyclobutylamino)methyl)-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.40 | -50.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Alectos Therapeutics Inc.; Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions were carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acet... | US Patent US9718854 (2017) BindingDB Entry DOI: 10.7270/Q2CJ8GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-2-oxoglutarate aldolase, mitochondrial (Homo sapiens (Human)) | BDBM267936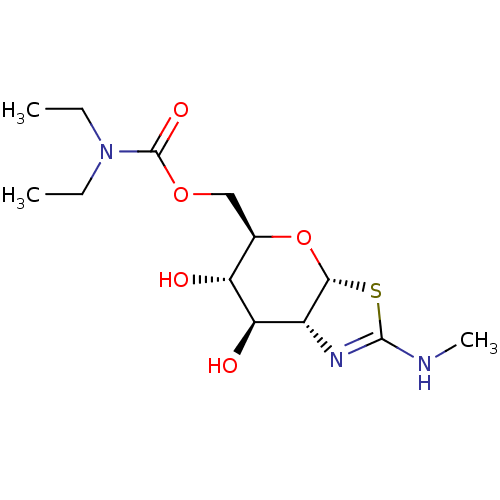 (((3aR,5R,6S,7R,7aR)-6,7-dihydroxy- 2-(methylamino)...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.70 | -48.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Alectos Therapeutics Inc.; Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions were carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acet... | US Patent US9718854 (2017) BindingDB Entry DOI: 10.7270/Q2CJ8GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-2-oxoglutarate aldolase, mitochondrial (Homo sapiens (Human)) | BDBM267938 ((3aR,5R,6S,7R,7aR)-2- (methylamino)-5- ((methylami...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.80 | -48.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Alectos Therapeutics Inc.; Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions were carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acet... | US Patent US9718854 (2017) BindingDB Entry DOI: 10.7270/Q2CJ8GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-2-oxoglutarate aldolase, mitochondrial (Homo sapiens (Human)) | BDBM267964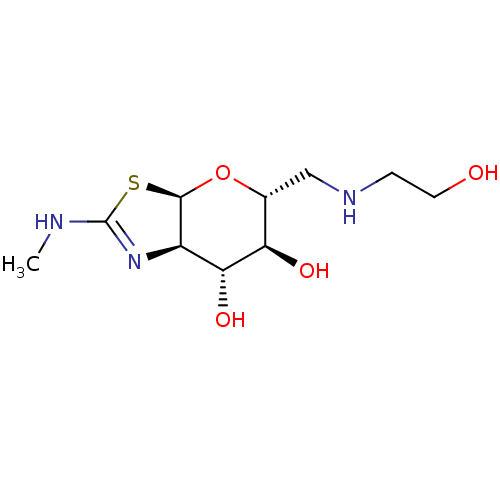 ((3aR,5R,6S,7R,7aR)-5-(((2- hydroxyethyl)amino)meth...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Alectos Therapeutics Inc.; Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions were carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acet... | US Patent US9718854 (2017) BindingDB Entry DOI: 10.7270/Q2CJ8GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-2-oxoglutarate aldolase, mitochondrial (Homo sapiens (Human)) | BDBM267943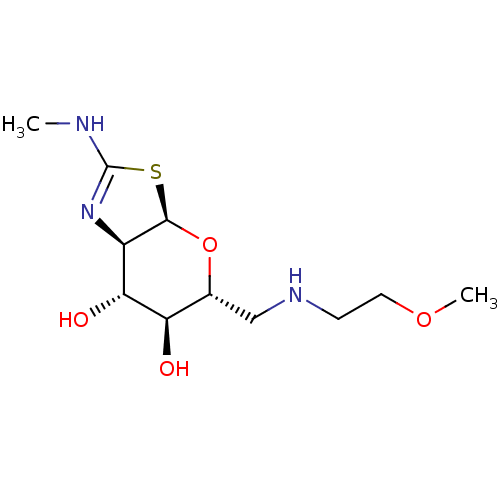 ((3aR,5R,6S,7R,7aR)-5-(((2- methoxyethyl)amino)meth...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.30 | -48.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Alectos Therapeutics Inc.; Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions were carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acet... | US Patent US9718854 (2017) BindingDB Entry DOI: 10.7270/Q2CJ8GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-2-oxoglutarate aldolase, mitochondrial (Homo sapiens (Human)) | BDBM267941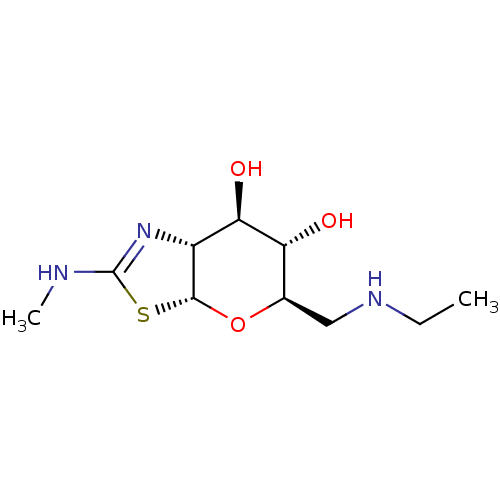 ((3aR,5R,6S,7R,7aR)-5- ((ethylamino)methyl)-2- (met...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.70 | -48.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Alectos Therapeutics Inc.; Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions were carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acet... | US Patent US9718854 (2017) BindingDB Entry DOI: 10.7270/Q2CJ8GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-2-oxoglutarate aldolase, mitochondrial (Homo sapiens (Human)) | BDBM267942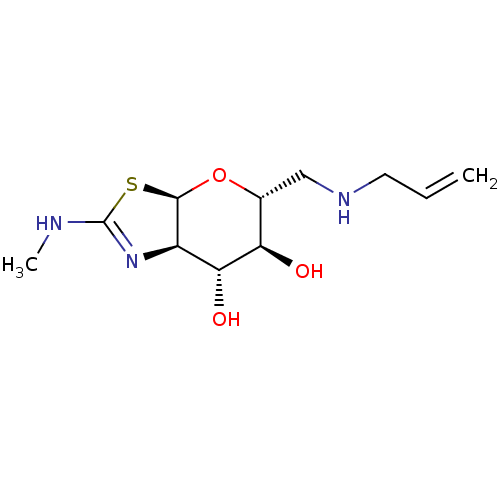 ((3aR,5R,6S,7R,7aR)-5- ((allylamino)methyl)-2- (met...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.80 | -48.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Alectos Therapeutics Inc.; Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions were carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acet... | US Patent US9718854 (2017) BindingDB Entry DOI: 10.7270/Q2CJ8GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-2-oxoglutarate aldolase, mitochondrial (Homo sapiens (Human)) | BDBM267965 (2-((((3aR,5R,6S,7R,7aR)-6,7- dihydroxy-2-(methylam...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.40 | -47.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Alectos Therapeutics Inc.; Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions were carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acet... | US Patent US9718854 (2017) BindingDB Entry DOI: 10.7270/Q2CJ8GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-2-oxoglutarate aldolase, mitochondrial (Homo sapiens (Human)) | BDBM267937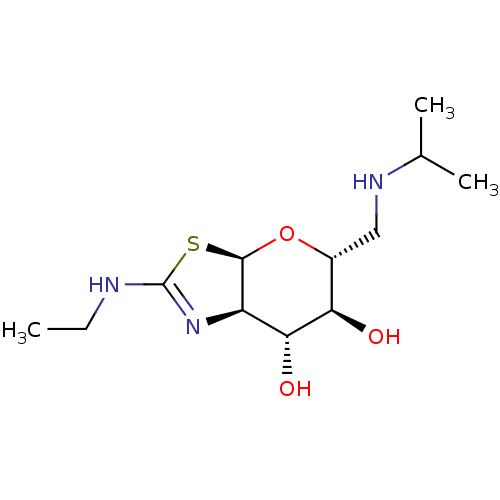 ((3aR,5R,6S,7R,7aR)-2-(ethylamino)- 5-((isopropylam...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.40 | -47.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Alectos Therapeutics Inc.; Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions were carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acet... | US Patent US9718854 (2017) BindingDB Entry DOI: 10.7270/Q2CJ8GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-2-oxoglutarate aldolase, mitochondrial (Homo sapiens (Human)) | BDBM267939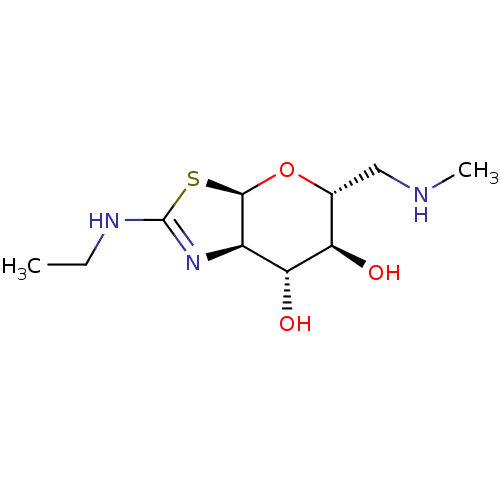 ((3aR,5R,6S,7R,7aR)-2-(ethylamino)- 5-((methylamino...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.60 | -47.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Alectos Therapeutics Inc.; Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions were carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acet... | US Patent US9718854 (2017) BindingDB Entry DOI: 10.7270/Q2CJ8GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-2-oxoglutarate aldolase, mitochondrial (Homo sapiens (Human)) | BDBM267945 ((3aR,5R,6S,7R,7aR)-5- ((cyclopropylamino)methyl)-2...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | -46.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Alectos Therapeutics Inc.; Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions were carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acet... | US Patent US9718854 (2017) BindingDB Entry DOI: 10.7270/Q2CJ8GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-2-oxoglutarate aldolase, mitochondrial (Homo sapiens (Human)) | BDBM267963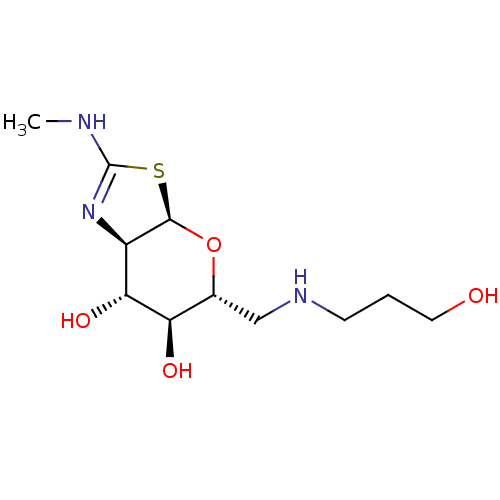 ((3aR,5R,6S,7R,7aR)-5-(((3- hydroxypropyl)amino)met...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | -46.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Alectos Therapeutics Inc.; Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions were carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acet... | US Patent US9718854 (2017) BindingDB Entry DOI: 10.7270/Q2CJ8GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-2-oxoglutarate aldolase, mitochondrial (Homo sapiens (Human)) | BDBM267962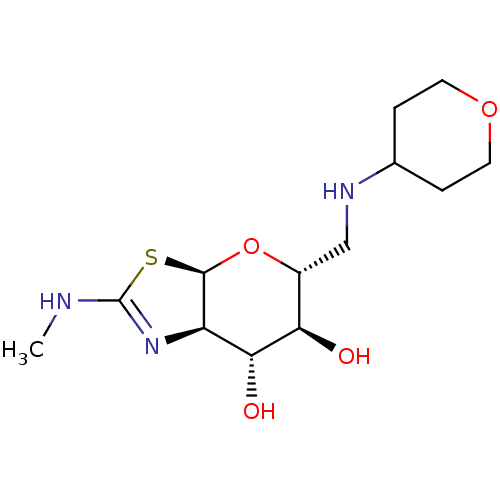 ((3aR,5R,6S,7R,7aR)-2- (methylamino)-5-(((tetrahydr...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.5 | -46.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Alectos Therapeutics Inc.; Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions were carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acet... | US Patent US9718854 (2017) BindingDB Entry DOI: 10.7270/Q2CJ8GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-2-oxoglutarate aldolase, mitochondrial (Homo sapiens (Human)) | BDBM267966 ((3aR,5R,6S,7R,7aR)-5-(((1H-pyrazol- 3-yl)amino)met...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.80 | -46.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Alectos Therapeutics Inc.; Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions were carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acet... | US Patent US9718854 (2017) BindingDB Entry DOI: 10.7270/Q2CJ8GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-2-oxoglutarate aldolase, mitochondrial (Homo sapiens (Human)) | BDBM267948 ((3aR,5R,6S,7R,7aR)-5- ((cyclohexylamino)methyl)-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9.30 | -45.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Alectos Therapeutics Inc.; Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions were carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acet... | US Patent US9718854 (2017) BindingDB Entry DOI: 10.7270/Q2CJ8GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-2-oxoglutarate aldolase, mitochondrial (Homo sapiens (Human)) | BDBM267784 (((3aR,5R,6S,7R,7aR)-6,7-dihydroxy- 2-(methylamino)...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9.90 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Alectos Therapeutics Inc.; Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions were carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acet... | US Patent US9718854 (2017) BindingDB Entry DOI: 10.7270/Q2CJ8GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-2-oxoglutarate aldolase, mitochondrial (Homo sapiens (Human)) | BDBM267968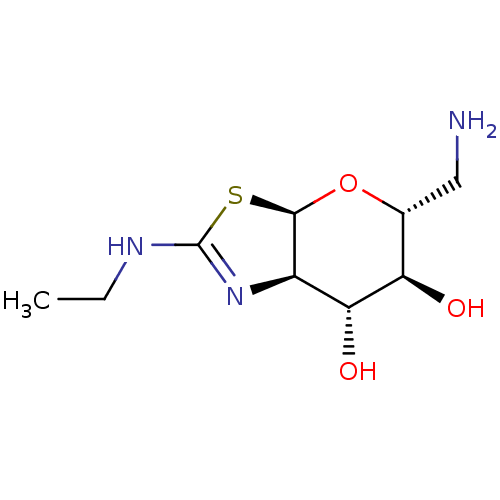 ((3aR,5R,6S,7R,7aR)-5 (aminomethyl)-2-(ethylamino)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 11 | -45.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Alectos Therapeutics Inc.; Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions were carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acet... | US Patent US9718854 (2017) BindingDB Entry DOI: 10.7270/Q2CJ8GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-2-oxoglutarate aldolase, mitochondrial (Homo sapiens (Human)) | BDBM267967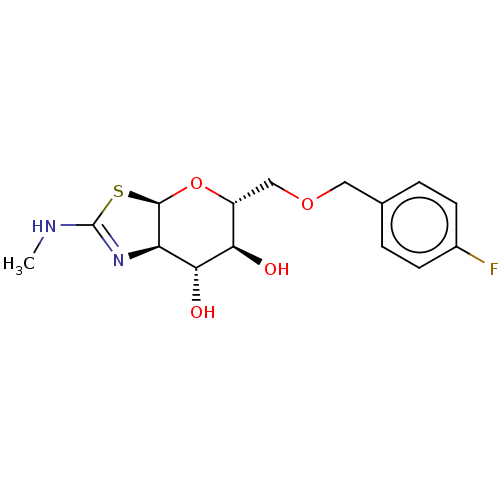 ((3aR,5R,6S,7R,7aR)-5-(((4- fluorobenzyl)oxy)methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 25 | -43.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Alectos Therapeutics Inc.; Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions were carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acet... | US Patent US9718854 (2017) BindingDB Entry DOI: 10.7270/Q2CJ8GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-2-oxoglutarate aldolase, mitochondrial (Homo sapiens (Human)) | BDBM50160721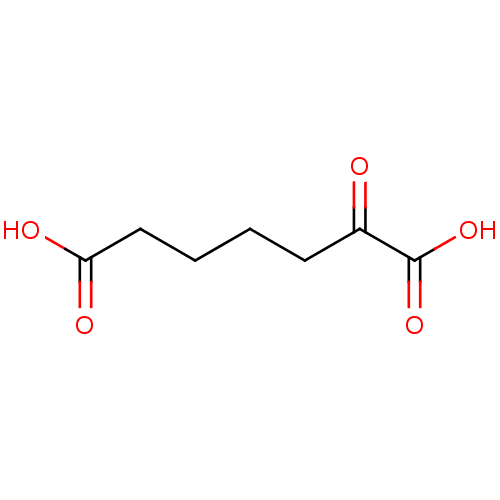 (2-Oxo-heptanedioic acid | 2-oxoheptanedioic acid |...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne Curated by ChEMBL | Assay Description Inhibition of dihydrodipicolinate synthase | Bioorg Med Chem Lett 18: 460-3 (2008) Article DOI: 10.1016/j.bmcl.2007.11.108 BindingDB Entry DOI: 10.7270/Q2QZ29QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-2-oxoglutarate aldolase, mitochondrial (Homo sapiens (Human)) | BDBM50229870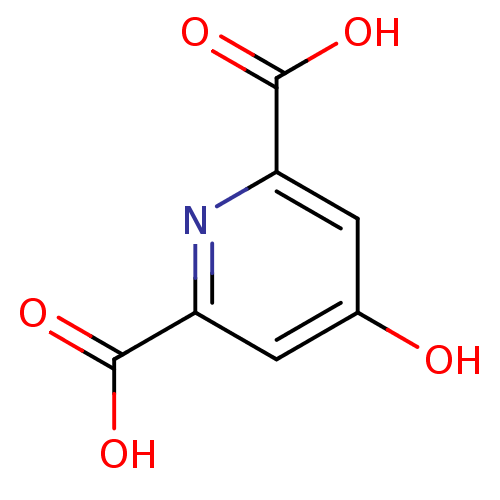 (4-oxo-1,4-dihydropyridine-2,6-dicarboxylic acid | ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne Curated by ChEMBL | Assay Description Inhibition of dihydrodipicolinate synthase | Bioorg Med Chem Lett 18: 460-3 (2008) Article DOI: 10.1016/j.bmcl.2007.11.108 BindingDB Entry DOI: 10.7270/Q2QZ29QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-2-oxoglutarate aldolase, mitochondrial (Homo sapiens (Human)) | BDBM50411597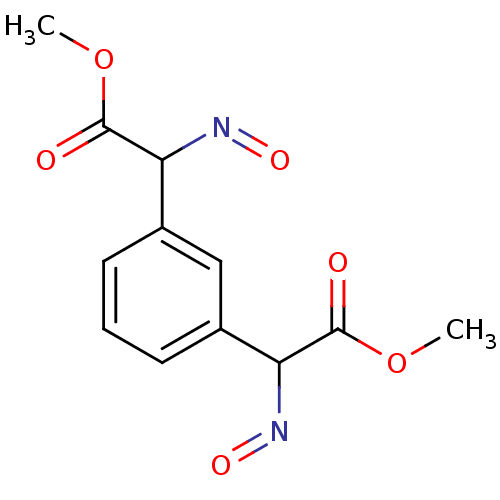 (CHEMBL403480) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne Curated by ChEMBL | Assay Description Inhibition of dihydrodipicolinate synthase | Bioorg Med Chem Lett 18: 460-3 (2008) Article DOI: 10.1016/j.bmcl.2007.11.108 BindingDB Entry DOI: 10.7270/Q2QZ29QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-2-oxoglutarate aldolase, mitochondrial (Homo sapiens (Human)) | BDBM50411596 (CHEMBL253062) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.96E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne Curated by ChEMBL | Assay Description Inhibition of dihydrodipicolinate synthase | Bioorg Med Chem Lett 18: 460-3 (2008) Article DOI: 10.1016/j.bmcl.2007.11.108 BindingDB Entry DOI: 10.7270/Q2QZ29QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-2-oxoglutarate aldolase, mitochondrial (Homo sapiens (Human)) | BDBM26116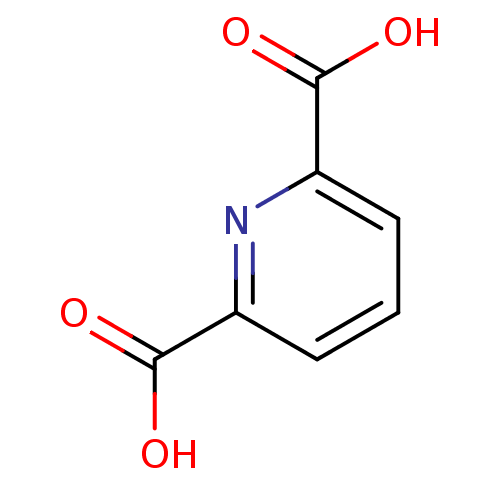 (CHEMBL284104 | Dipicolinate | pyridine carboxylate...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.10E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Melbourne Curated by ChEMBL | Assay Description Inhibition of dihydrodipicolinate synthase | Bioorg Med Chem Lett 18: 460-3 (2008) Article DOI: 10.1016/j.bmcl.2007.11.108 BindingDB Entry DOI: 10.7270/Q2QZ29QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||