

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mast cell carboxypeptidase A (Rattus norvegicus) | BDBM50530230 (CHEMBL4445882) | MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of rat CPA3 using AAFP as substrate preincubated for 45 mins measured at 30 sec intervals for 15 mins by UV/vis-spectrophotometry | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast cell carboxypeptidase A (Rattus norvegicus) | BDBM50530230 (CHEMBL4445882) | MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona Curated by ChEMBL | Assay Description Inhibition of rat CPA3 using AAFP as substrate preincubated for 45 mins measured at 30 sec intervals for 15 mins by UV/vis-spectrophotometry | J Med Chem 62: 1917-1931 (2019) Article DOI: 10.1021/acs.jmedchem.8b01465 BindingDB Entry DOI: 10.7270/Q2K077R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast cell carboxypeptidase A (Homo sapiens (Human)) | BDBM50281176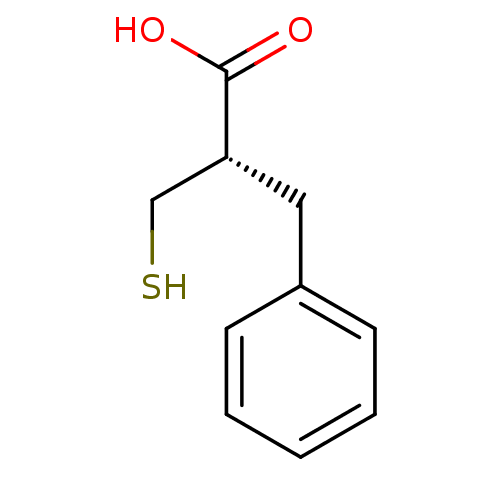 ((S)-2-Mercaptomethyl-3-phenyl-propionic acid | 2-T...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid UniChem Similars | Article | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against carboxypeptidase A | Bioorg Med Chem Lett 3: 2681-2684 (1993) Article DOI: 10.1016/S0960-894X(01)80741-1 BindingDB Entry DOI: 10.7270/Q2GM876S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast cell carboxypeptidase A (Homo sapiens (Human)) | BDBM50121929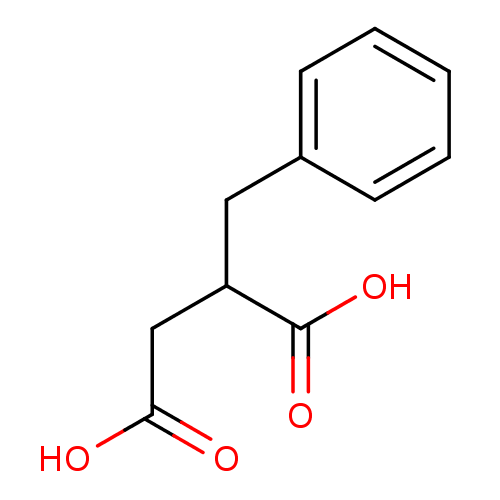 ((phenylmethyl)butanedioic acid | 2-benzylbutanedio...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was evaluated for the inhibition of Carboxypeptidase A | J Med Chem 45: 5609-16 (2002) BindingDB Entry DOI: 10.7270/Q270825W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast cell carboxypeptidase A (Homo sapiens (Human)) | BDBM50121917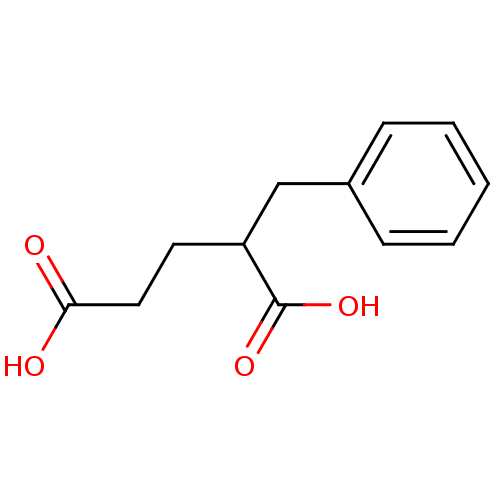 (2-Benzyl-pentanedioic acid | CHEMBL153232) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description The compound was evaluated for the inhibition of Carboxypeptidase A | J Med Chem 45: 5609-16 (2002) BindingDB Entry DOI: 10.7270/Q270825W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast cell carboxypeptidase A (Homo sapiens (Human)) | BDBM50058391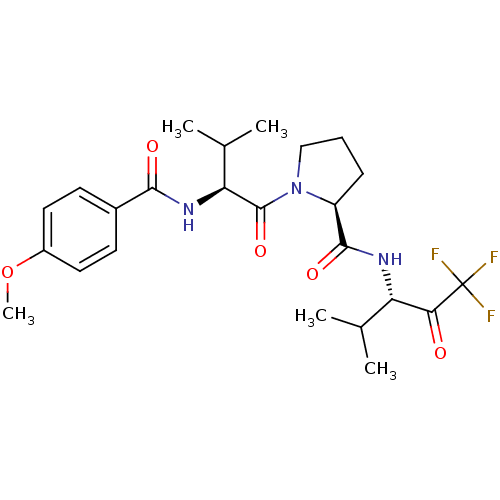 ((S)-1-[(S)-2-(4-Methoxy-benzoylamino)-3-methyl-but...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity of the compound for carboxypeptidase A | J Med Chem 40: 3173-81 (1997) Article DOI: 10.1021/jm970250z BindingDB Entry DOI: 10.7270/Q2GT5M85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast cell carboxypeptidase A (Homo sapiens (Human)) | BDBM50188513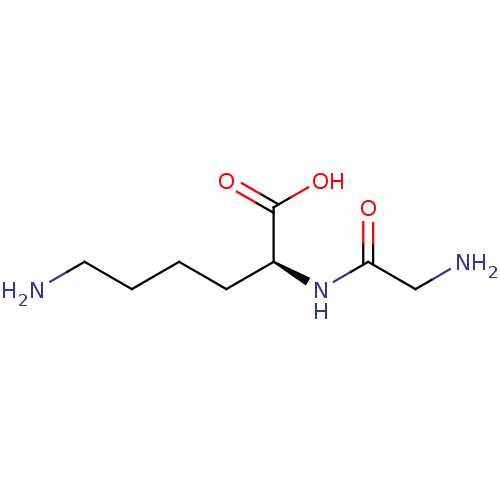 ((S)-6-Amino-2-(2-amino-acetylamino)-hexanoic acid ...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 1.29E+5 | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Binding affinity against Carboxypeptidase A | J Med Chem 45: 2770-80 (2002) Article DOI: 10.1021/jm0105833 BindingDB Entry DOI: 10.7270/Q2MG7S8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast cell carboxypeptidase A (Homo sapiens (Human)) | BDBM50473781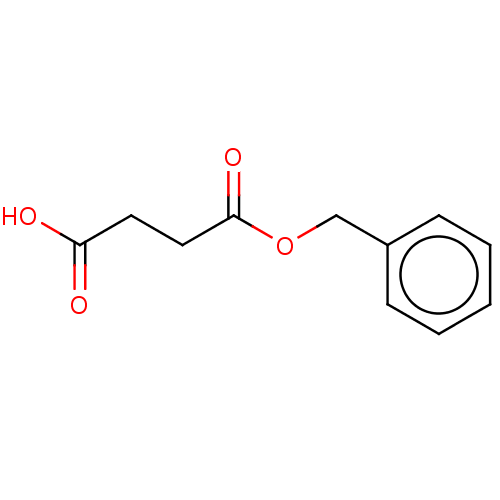 (Succinic acid monobenzyl ester) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 447 | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Binding affinity against Carboxypeptidase A | J Med Chem 45: 2770-80 (2002) Article DOI: 10.1021/jm0105833 BindingDB Entry DOI: 10.7270/Q2MG7S8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||