 Found 2459 hits Enz. Inhib. hit(s) with Target = 'Nitric Oxide Synthase, inducible'
Found 2459 hits Enz. Inhib. hit(s) with Target = 'Nitric Oxide Synthase, inducible' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nitric oxide synthase, inducible
(Rattus norvegicus) | BDBM50386178
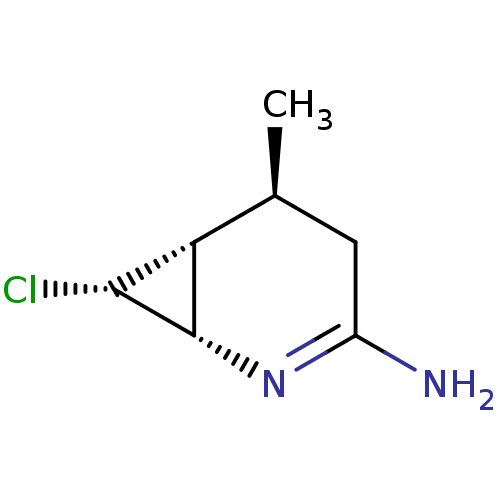
(CHEMBL1800346 | ONO-1714)Show SMILES C[C@H]1CC(N)=N[C@@H]2[C@H](Cl)[C@H]12 |r,c:4| Show InChI InChI=1S/C7H11ClN2/c1-3-2-4(9)10-7-5(3)6(7)8/h3,5-7H,2H2,1H3,(H2,9,10)/t3-,5?,6+,7-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Cajal (CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of rat iNOS |
Eur J Med Chem 54: 439-46 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.031
BindingDB Entry DOI: 10.7270/Q2NC6280 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50061231
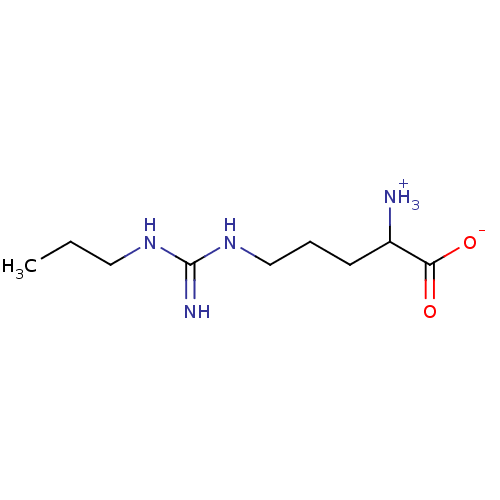
(2-Amino-5-(N'-propyl-guanidino)-pentanoic acid | C...)Show InChI InChI=1S/C9H20N4O2/c1-2-5-12-9(11)13-6-3-4-7(10)8(14)15/h7H,2-6,10H2,1H3,(H,14,15)(H3,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against recombinant inducible nitric oxide synthase (iNOS) from mouse |
J Med Chem 40: 3869-70 (1998)
Article DOI: 10.1021/jm970550g
BindingDB Entry DOI: 10.7270/Q2C829ZT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50386178

(CHEMBL1800346 | ONO-1714)Show SMILES C[C@H]1CC(N)=N[C@@H]2[C@H](Cl)[C@H]12 |r,c:4| Show InChI InChI=1S/C7H11ClN2/c1-3-2-4(9)10-7-5(3)6(7)8/h3,5-7H,2H2,1H3,(H2,9,10)/t3-,5?,6+,7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto Cajal (CSIC)
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS |
Eur J Med Chem 54: 439-46 (2012)
Article DOI: 10.1016/j.ejmech.2012.05.031
BindingDB Entry DOI: 10.7270/Q2NC6280 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50386178

(CHEMBL1800346 | ONO-1714)Show SMILES C[C@H]1CC(N)=N[C@@H]2[C@H](Cl)[C@H]12 |r,c:4| Show InChI InChI=1S/C7H11ClN2/c1-3-2-4(9)10-7-5(3)6(7)8/h3,5-7H,2H2,1H3,(H2,9,10)/t3-,5?,6+,7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50111438
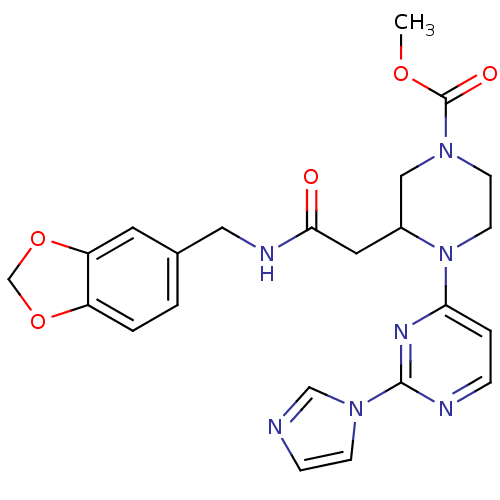
(3-{[(Benzo[1,3]dioxol-5-ylmethyl)-carbamoyl]-methy...)Show SMILES COC(=O)N1CCN(C(CC(=O)NCc2ccc3OCOc3c2)C1)c1ccnc(n1)-n1ccnc1 Show InChI InChI=1S/C23H25N7O5/c1-33-23(32)28-8-9-30(20-4-5-25-22(27-20)29-7-6-24-14-29)17(13-28)11-21(31)26-12-16-2-3-18-19(10-16)35-15-34-18/h2-7,10,14,17H,8-9,11-13,15H2,1H3,(H,26,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding against the partially purified human Inducible nitric oxide synthase |
J Med Chem 45: 1543-58 (2002)
BindingDB Entry DOI: 10.7270/Q2CN74ND |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50330882
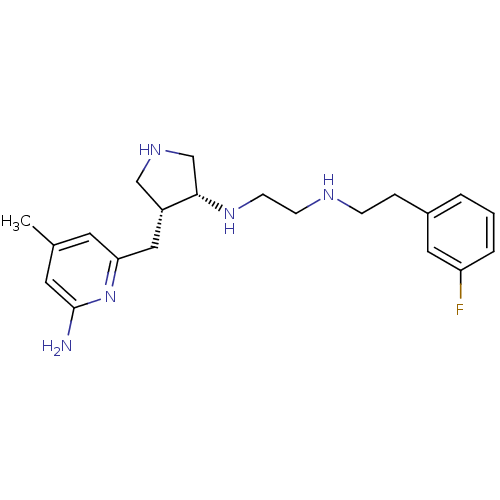
(CHEMBL1277951 | CHEMBL594682 | N1-((3R,4R)-4-((6-a...)Show SMILES Cc1cc(N)nc(C[C@@H]2CNC[C@@H]2NCCNCCc2cccc(F)c2)c1 |r| Show InChI InChI=1S/C21H30FN5/c1-15-9-19(27-21(23)10-15)12-17-13-25-14-20(17)26-8-7-24-6-5-16-3-2-4-18(22)11-16/h2-4,9-11,17,20,24-26H,5-8,12-14H2,1H3,(H2,23,27)/t17-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50055281
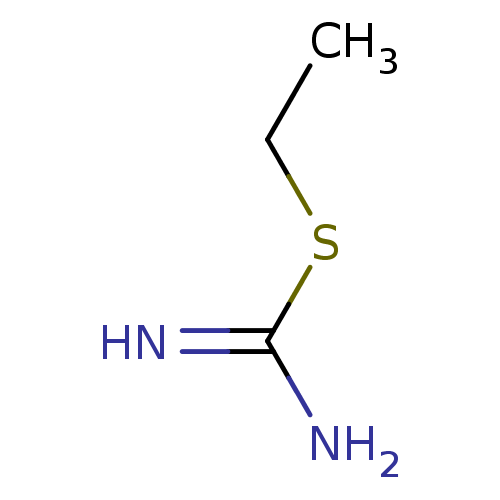
(2-Ethyl-isothiourea | CHEMBL321691 | ETHYLISOTHIOU...)Show InChI InChI=1S/C3H8N2S/c1-2-6-3(4)5/h2H2,1H3,(H3,4,5) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University Medical School
Curated by ChEMBL
| Assay Description
Binding affinity against mouse Inducible nitric oxide synthase (iNOS) |
J Med Chem 39: 5110-8 (1997)
Article DOI: 10.1021/jm960481q
BindingDB Entry DOI: 10.7270/Q29W0DKZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50328814
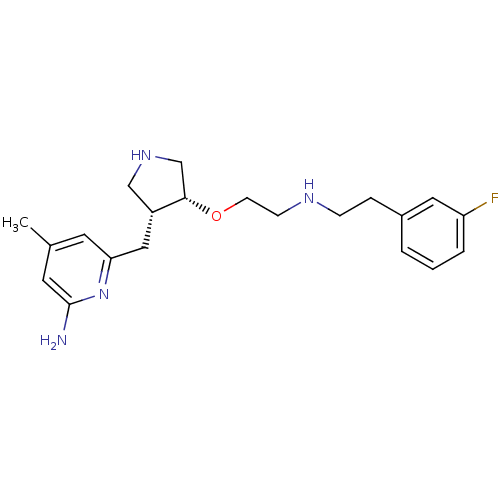
((+/-)-6-((4-(2-(3-fluorophenethylamino)ethoxy)pyrr...)Show SMILES Cc1cc(N)nc(C[C@@H]2CNC[C@@H]2OCCNCCc2cccc(F)c2)c1 |r| Show InChI InChI=1S/C21H29FN4O/c1-15-9-19(26-21(23)10-15)12-17-13-25-14-20(17)27-8-7-24-6-5-16-3-2-4-18(22)11-16/h2-4,9-11,17,20,24-25H,5-8,12-14H2,1H3,(H2,23,26)/t17-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50255365
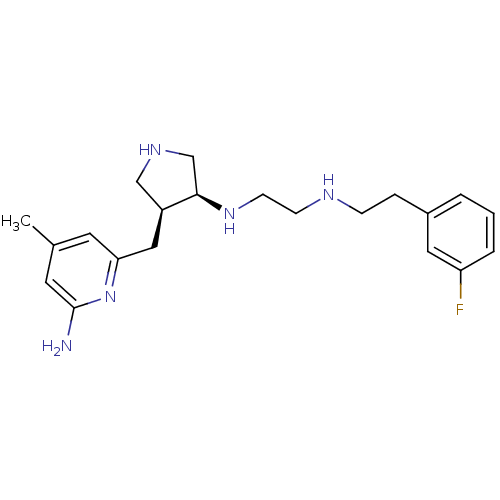
((+/-)-cis-N1-((3S,4S)-4-((6-amino-4-methylpyridin-...)Show SMILES Cc1cc(N)nc(C[C@H]2CNC[C@H]2NCCNCCc2cccc(F)c2)c1 |r| Show InChI InChI=1S/C21H30FN5/c1-15-9-19(27-21(23)10-15)12-17-13-25-14-20(17)26-8-7-24-6-5-16-3-2-4-18(22)11-16/h2-4,9-11,17,20,24-26H,5-8,12-14H2,1H3,(H2,23,27)/t17-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50278675

((+/-)-cis-6-((4-(2-(3-Fluorophenethylamino)-ethoxy...)Show SMILES Cc1cc(N)nc(C[C@H]2CNC[C@H]2OCCNCCc2cccc(F)c2)c1 |r| Show InChI InChI=1S/C21H29FN4O/c1-15-9-19(26-21(23)10-15)12-17-13-25-14-20(17)27-8-7-24-6-5-16-3-2-4-18(22)11-16/h2-4,9-11,17,20,24-25H,5-8,12-14H2,1H3,(H2,23,26)/t17-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50330883
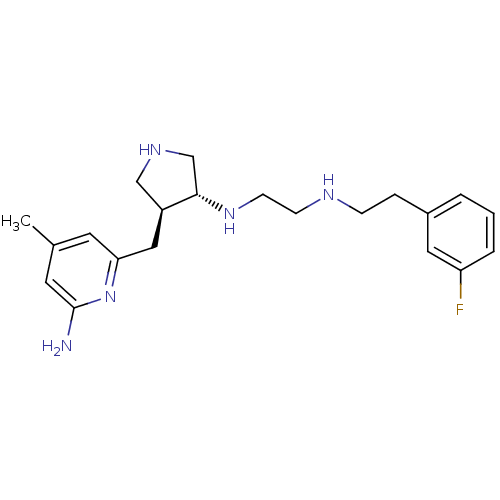
(CHEMBL1233715 | CHEMBL1277952 | N1-((3R,4S)-4-((6-...)Show SMILES Cc1cc(N)nc(C[C@H]2CNC[C@@H]2NCCNCCc2cccc(F)c2)c1 |r| Show InChI InChI=1S/C21H30FN5/c1-15-9-19(27-21(23)10-15)12-17-13-25-14-20(17)26-8-7-24-6-5-16-3-2-4-18(22)11-16/h2-4,9-11,17,20,24-26H,5-8,12-14H2,1H3,(H2,23,27)/t17-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50065823
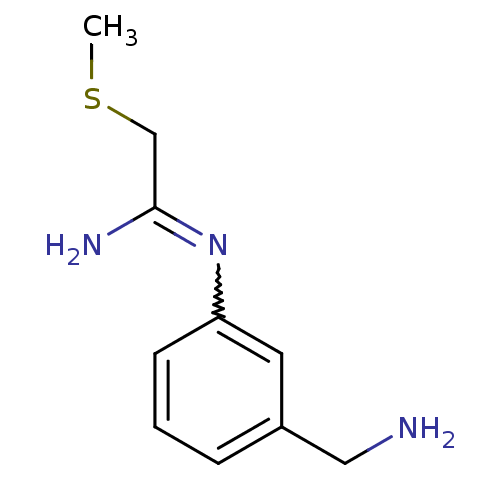
(CHEMBL555794 | N-(3-Aminomethyl-phenyl)-2-methylsu...)Show InChI InChI=1S/C10H15N3S/c1-14-7-10(12)13-9-4-2-3-8(5-9)6-11/h2-5H,6-7,11H2,1H3,(H2,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50330875

(CHEMBL1233717 | CHEMBL1278037 | N-{(3S,4R)-4-[(6-a...)Show SMILES Cc1cc(N)nc(C[C@@H]2CNC[C@H]2NCCNCCc2cccc(F)c2)c1 |r| Show InChI InChI=1S/C21H30FN5/c1-15-9-19(27-21(23)10-15)12-17-13-25-14-20(17)26-8-7-24-6-5-16-3-2-4-18(22)11-16/h2-4,9-11,17,20,24-26H,5-8,12-14H2,1H3,(H2,23,27)/t17-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50330897
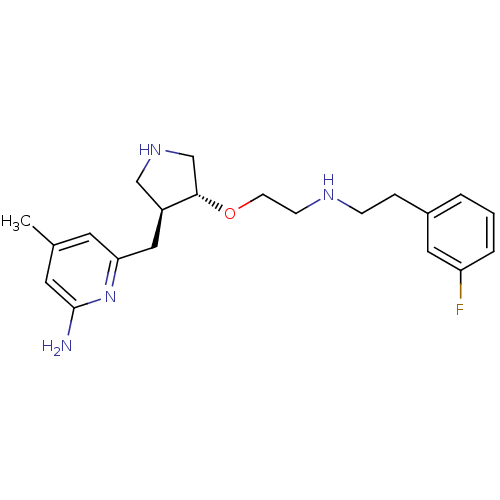
(6-(((3R,4S)-4-(2-(4-fluorobenzylamino)ethoxy)pyrro...)Show SMILES Cc1cc(N)nc(C[C@H]2CNC[C@@H]2OCCNCCc2cccc(F)c2)c1 |r| Show InChI InChI=1S/C21H29FN4O/c1-15-9-19(26-21(23)10-15)12-17-13-25-14-20(17)27-8-7-24-6-5-16-3-2-4-18(22)11-16/h2-4,9-11,17,20,24-25H,5-8,12-14H2,1H3,(H2,23,26)/t17-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM29234
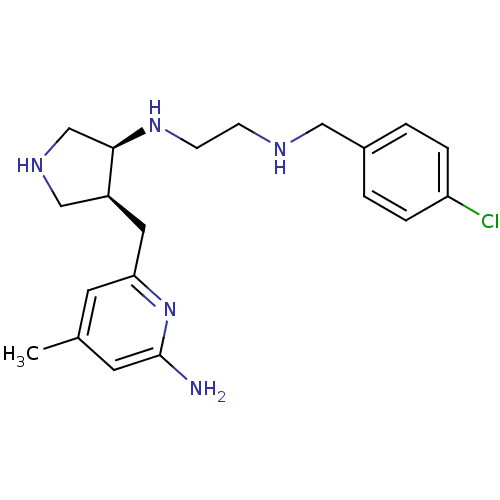
(CHEMBL481815 | US9090589, 6 | aminopyridine-pyrrol...)Show SMILES Cc1cc(N)nc(C[C@H]2CNC[C@H]2NCCNCc2ccc(Cl)cc2)c1 |r| Show InChI InChI=1S/C20H28ClN5/c1-14-8-18(26-20(22)9-14)10-16-12-24-13-19(16)25-7-6-23-11-15-2-4-17(21)5-3-15/h2-5,8-9,16,19,23-25H,6-7,10-13H2,1H3,(H2,22,26)/t16-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50330873
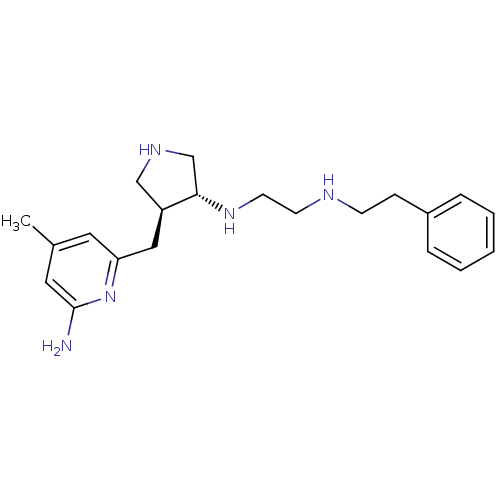
(CHEMBL1277786 | US9090589, 4 | trans rac-N1-(4-((6...)Show SMILES Cc1cc(N)nc(C[C@H]2CNC[C@@H]2NCCNCCc2ccccc2)c1 |r| Show InChI InChI=1S/C21H31N5/c1-16-11-19(26-21(22)12-16)13-18-14-24-15-20(18)25-10-9-23-8-7-17-5-3-2-4-6-17/h2-6,11-12,18,20,23-25H,7-10,13-15H2,1H3,(H2,22,26)/t18-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50064015
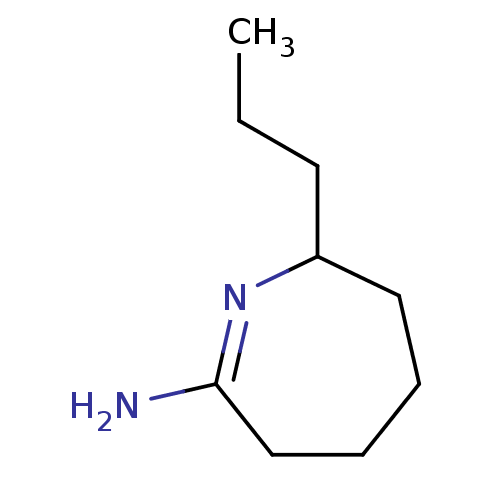
(7-Propyl-azepan-(2Z)-ylideneamine; hydrochloride |...)Show InChI InChI=1S/C9H18N2/c1-2-5-8-6-3-4-7-9(10)11-8/h8H,2-7H2,1H3,(H2,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against L-arginine binding to Inducible nitric oxide synthase |
J Med Chem 41: 1361-6 (1998)
Article DOI: 10.1021/jm9704715
BindingDB Entry DOI: 10.7270/Q2348M29 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50330874
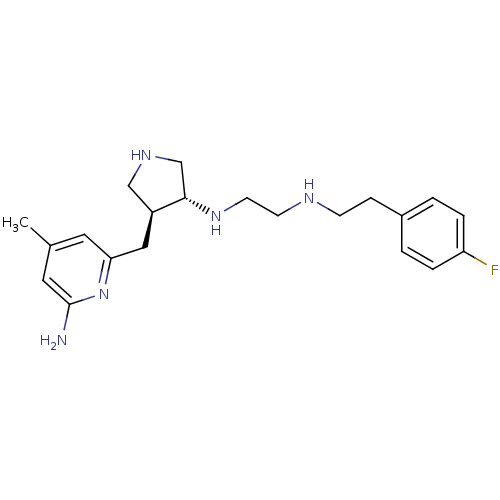
(CHEMBL1277787 | trans rac-N1-(4-((6-amino-4-methyl...)Show SMILES Cc1cc(N)nc(C[C@H]2CNC[C@@H]2NCCNCCc2ccc(F)cc2)c1 |r| Show InChI InChI=1S/C21H30FN5/c1-15-10-19(27-21(23)11-15)12-17-13-25-14-20(17)26-9-8-24-7-6-16-2-4-18(22)5-3-16/h2-5,10-11,17,20,24-26H,6-9,12-14H2,1H3,(H2,23,27)/t17-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50095172

(CHEMBL262040 | N-(3-Aminomethyl-phenyl)-thiophene-...)Show InChI InChI=1S/C12H13N3S/c13-8-9-3-1-4-10(7-9)15-12(14)11-5-2-6-16-11/h1-7H,8,13H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition against induced Nitric Oxide Synthase(iNOS) |
Bioorg Med Chem Lett 10: 2771-4 (2000)
BindingDB Entry DOI: 10.7270/Q23B60NS |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50065843
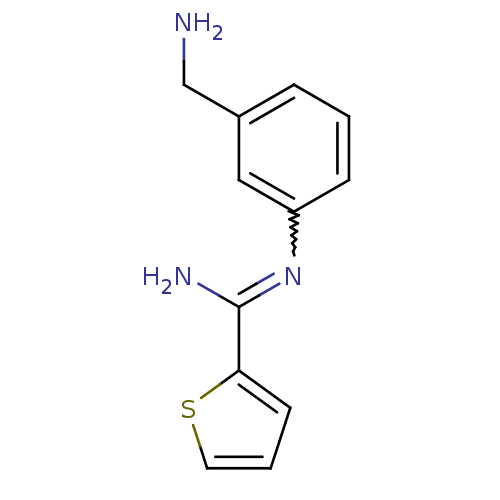
(CHEMBL553081 | CHEMBL555715 | N-(3-Aminomethyl-phe...)Show InChI InChI=1S/C12H13N3S/c13-8-9-3-1-4-10(7-9)15-12(14)11-5-2-6-16-11/h1-7H,8,13H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50065805
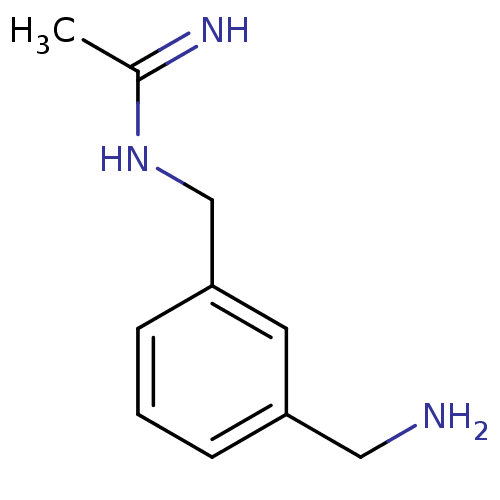
(CHEMBL544788 | N-(3-Aminomethyl-benzyl)-acetamidin...)Show InChI InChI=1S/C10H15N3/c1-8(12)13-7-10-4-2-3-9(5-10)6-11/h2-5H,6-7,11H2,1H3,(H2,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50095181
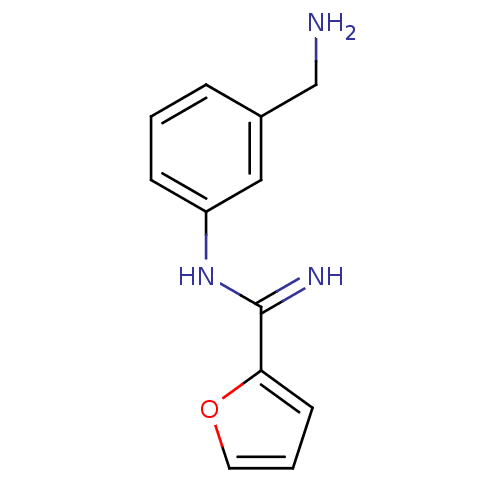
(CHEMBL96680 | N-(3-Aminomethyl-phenyl)-furan-2-car...)Show InChI InChI=1S/C12H13N3O/c13-8-9-3-1-4-10(7-9)15-12(14)11-5-2-6-16-11/h1-7H,8,13H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition against induced Nitric Oxide Synthase(iNOS) |
Bioorg Med Chem Lett 10: 2771-4 (2000)
BindingDB Entry DOI: 10.7270/Q23B60NS |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50065807
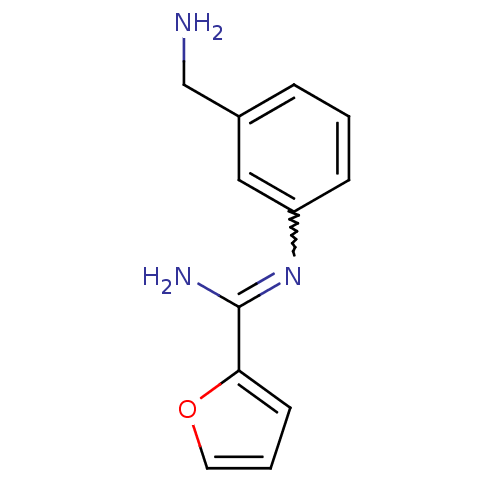
(CHEMBL552825 | N-(3-Aminomethyl-phenyl)-furan-2-ca...)Show InChI InChI=1S/C12H13N3O/c13-8-9-3-1-4-10(7-9)15-12(14)11-5-2-6-16-11/h1-7H,8,13H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM152698

((S)-2-Amino-5-formimidamidopentanoic acid (7))Show InChI InChI=1S/C6H13N3O2/c7-4-9-3-1-2-5(8)6(10)11/h4-5H,1-3,8H2,(H2,7,9)(H,10,11)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 180 | n/a | 400 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Northwestern University
| Assay Description
The assay mixture contained L-arginine (10 μM), NADPH (100 μM), oxyhemoglobin (0.125 mg/mL), tetrahydrobiopterin (10 μM), and differen... |
Biochemistry 54: 2530-8 (2015)
Article DOI: 10.1021/acs.biochem.5b00135
BindingDB Entry DOI: 10.7270/Q23B5XWQ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)-Homo sapiens (Human)) | BDBM50136951
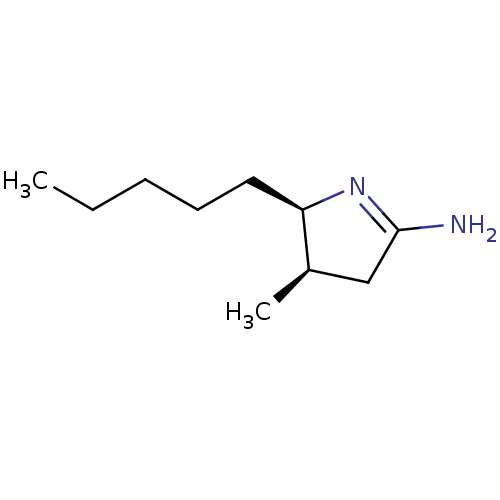
((4R,5R)-4-Methyl-5-pentyl-pyrrolidin-(2E)-ylidenea...)Show InChI InChI=1S/C10H20N2/c1-3-4-5-6-9-8(2)7-10(11)12-9/h8-9H,3-7H2,1-2H3,(H2,11,12)/t8-,9-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Inducible nitric oxide synthase |
J Med Chem 46: 5700-11 (2003)
Checked by Author
Article DOI: 10.1021/jm030301u
BindingDB Entry DOI: 10.7270/Q26D5TQV |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM29235
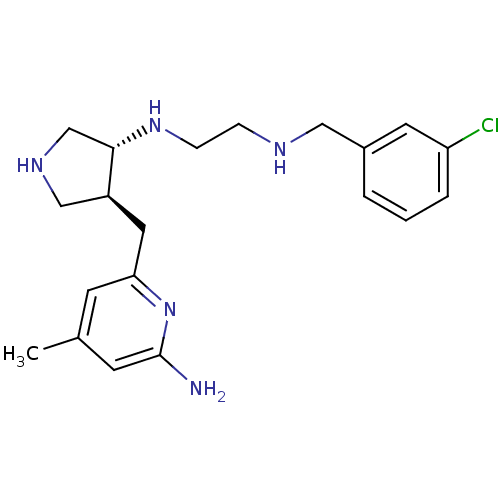
(aminopyridine-pyrrolidine, 7)Show SMILES Cc1cc(N)nc(C[C@H]2CNC[C@@H]2NCCNCc2cccc(Cl)c2)c1 |r| Show InChI InChI=1S/C20H28ClN5/c1-14-7-18(26-20(22)8-14)10-16-12-24-13-19(16)25-6-5-23-11-15-3-2-4-17(21)9-15/h2-4,7-9,16,19,23-25H,5-6,10-13H2,1H3,(H2,22,26)/t16-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50330882

(CHEMBL1277951 | CHEMBL594682 | N1-((3R,4R)-4-((6-a...)Show SMILES Cc1cc(N)nc(C[C@@H]2CNC[C@@H]2NCCNCCc2cccc(F)c2)c1 |r| Show InChI InChI=1S/C21H30FN5/c1-15-9-19(27-21(23)10-15)12-17-13-25-14-20(17)26-8-7-24-6-5-16-3-2-4-18(22)11-16/h2-4,9-11,17,20,24-26H,5-8,12-14H2,1H3,(H2,23,27)/t17-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| US Patent
| 273 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
US Patent
| Assay Description
Rat and human nNOS, murine macrophage iNOS, and human eNOS were recombinant enzymes (expressed in E. coli and purified as reported previously in the ... |
US Patent US9783500 (2017)
BindingDB Entry DOI: 10.7270/Q2930WBP |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50072297
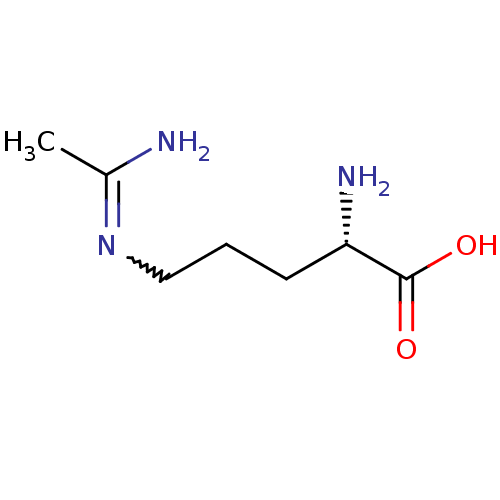
((S)-5-Acetimidoylamino-2-amino-pent | (S)-5-Acetim...)Show InChI InChI=1S/C7H15N3O2/c1-5(8)10-4-2-3-6(9)7(11)12/h6H,2-4,9H2,1H3,(H2,8,10)(H,11,12)/t6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory constant for the inhibition of human Inducible nitric oxide synthase |
Bioorg Med Chem Lett 7: 1763-1768 (1997)
Article DOI: 10.1016/S0960-894X(97)00309-0
BindingDB Entry DOI: 10.7270/Q2TQ61J5 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50438638

(CHEMBL2414426)Show InChI InChI=1S/C17H24N4/c1-12-8-14(20-16(18)10-12)6-4-3-5-7-15-9-13(2)11-17(19)21-15/h8-11H,3-7H2,1-2H3,(H2,18,20)(H2,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 375 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mouse macrophage iNOS expressed in Escherichia coli using L-arginine as substrate assessed as formation of nitric oxide mea... |
Bioorg Med Chem 21: 5323-31 (2013)
Article DOI: 10.1016/j.bmc.2013.06.014
BindingDB Entry DOI: 10.7270/Q2PZ5B7N |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM29233

(CHEMBL508014 | aminopyridine-pyrrolidine, 5)Show InChI InChI=1S/C12H21N5/c13-4-5-16-11-8-15-7-9(11)6-10-2-1-3-12(14)17-10/h1-3,9,11,15-16H,4-8,13H2,(H2,14,17)/t9-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 388 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50330869
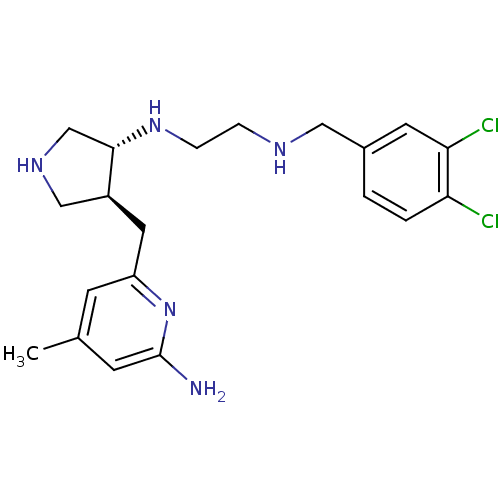
(CHEMBL1277601 | trans rac-N1-(4-((6-amino-4-methyl...)Show SMILES Cc1cc(N)nc(C[C@H]2CNC[C@@H]2NCCNCc2ccc(Cl)c(Cl)c2)c1 |r| Show InChI InChI=1S/C20H27Cl2N5/c1-13-6-16(27-20(23)7-13)9-15-11-25-12-19(15)26-5-4-24-10-14-2-3-17(21)18(22)8-14/h2-3,6-8,15,19,24-26H,4-5,9-12H2,1H3,(H2,23,27)/t15-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 414 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50065813

(CHEMBL555584 | N-(3-Aminomethyl-phenyl)-2-fluoro-a...)Show InChI InChI=1S/C9H12FN3/c10-5-9(12)13-8-3-1-2-7(4-8)6-11/h1-4H,5-6,11H2,(H2,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50330870
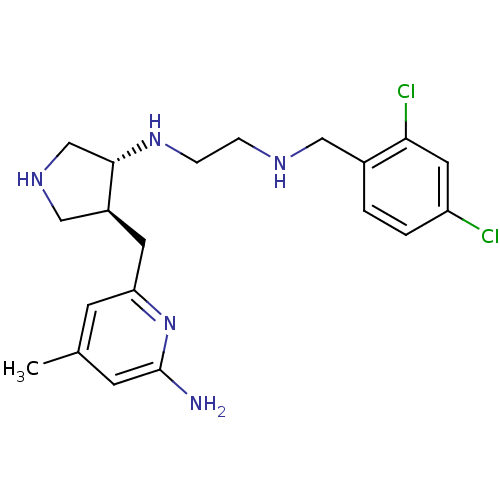
(CHEMBL1277602 | trans rac-N1-(4-((6-amino-4-methyl...)Show SMILES Cc1cc(N)nc(C[C@H]2CNC[C@@H]2NCCNCc2ccc(Cl)cc2Cl)c1 |r| Show InChI InChI=1S/C20H27Cl2N5/c1-13-6-17(27-20(23)7-13)8-15-11-25-12-19(15)26-5-4-24-10-14-2-3-16(21)9-18(14)22/h2-3,6-7,9,15,19,24-26H,4-5,8,10-12H2,1H3,(H2,23,27)/t15-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 483 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50330895

(CHEMBL1277327 | trans rac-N1-(4-((6-amino-4-methyl...)Show SMILES Cc1cc(N)nc(C[C@H]2CNC[C@@H]2NCCNCc2ccc(Cl)cc2)c1 |r| Show InChI InChI=1S/C20H28ClN5/c1-14-8-18(26-20(22)9-14)10-16-12-24-13-19(16)25-7-6-23-11-15-2-4-17(21)5-3-15/h2-5,8-9,16,19,23-25H,6-7,10-13H2,1H3,(H2,22,26)/t16-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50225106

((2S)-2-amino-5-{[(E)-amino(nitroimino)methyl]amino...)Show SMILES N[C@@H](CCCNC(N)=N[N+]([O-])=O)C(O)=O |r,w:8.8| Show InChI InChI=1S/C6H13N5O4/c7-4(5(12)13)2-1-3-9-6(8)10-11(14)15/h4H,1-3,7H2,(H,12,13)(H3,8,9,10)/t4-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50330882

(CHEMBL1277951 | CHEMBL594682 | N1-((3R,4R)-4-((6-a...)Show SMILES Cc1cc(N)nc(C[C@@H]2CNC[C@@H]2NCCNCCc2cccc(F)c2)c1 |r| Show InChI InChI=1S/C21H30FN5/c1-15-9-19(27-21(23)10-15)12-17-13-25-14-20(17)26-8-7-24-6-5-16-3-2-4-18(22)11-16/h2-4,9-11,17,20,24-26H,5-8,12-14H2,1H3,(H2,23,27)/t17-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| US Patent
| 581 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
US Patent
| Assay Description
Rat and human nNOS, murine macrophage iNOS, and human eNOS were recombinant enzymes (expressed in E. coli and purified as reported previously in the ... |
US Patent US9783500 (2017)
BindingDB Entry DOI: 10.7270/Q2930WBP |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50330871
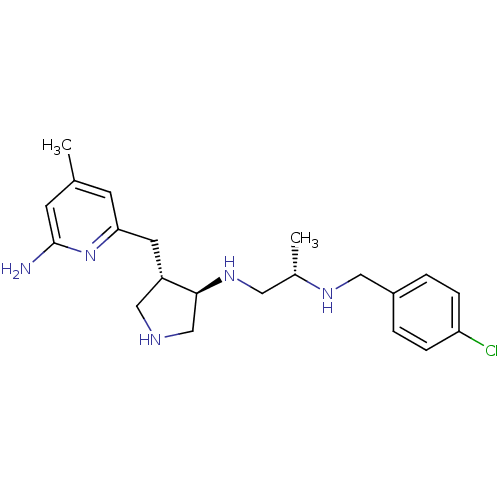
(CHEMBL1277693 | trans rac-(S)-N1-(4-((6-amino-4-me...)Show SMILES C[C@@H](CN[C@H]1CNC[C@@H]1Cc1cc(C)cc(N)n1)NCc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H30ClN5/c1-14-7-19(27-21(23)8-14)9-17-12-24-13-20(17)26-10-15(2)25-11-16-3-5-18(22)6-4-16/h3-8,15,17,20,24-26H,9-13H2,1-2H3,(H2,23,27)/t15-,17-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 589 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50289681
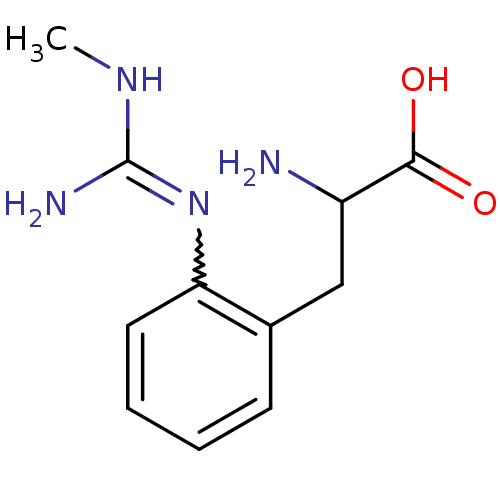
(2-Amino-3-[2-(N'-methyl-guanidino)-phenyl]-propion...)Show InChI InChI=1S/C11H16N4O2/c1-14-11(13)15-9-5-3-2-4-7(9)6-8(12)10(16)17/h2-5,8H,6,12H2,1H3,(H,16,17)(H3,13,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory constant for the inhibition of human Inducible nitric oxide synthase |
Bioorg Med Chem Lett 7: 1763-1768 (1997)
Article DOI: 10.1016/S0960-894X(97)00309-0
BindingDB Entry DOI: 10.7270/Q2TQ61J5 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50330862
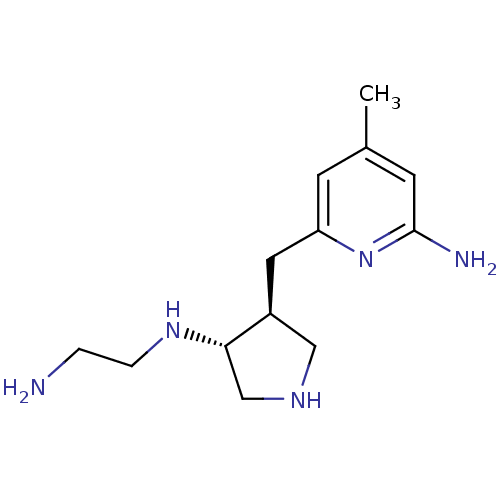
(CHEMBL1276118 | CHEMBL1276167 | trans rac-N1-(4-((...)Show InChI InChI=1S/C13H23N5/c1-9-4-11(18-13(15)5-9)6-10-7-16-8-12(10)17-3-2-14/h4-5,10,12,16-17H,2-3,6-8,14H2,1H3,(H2,15,18)/t10-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50061232
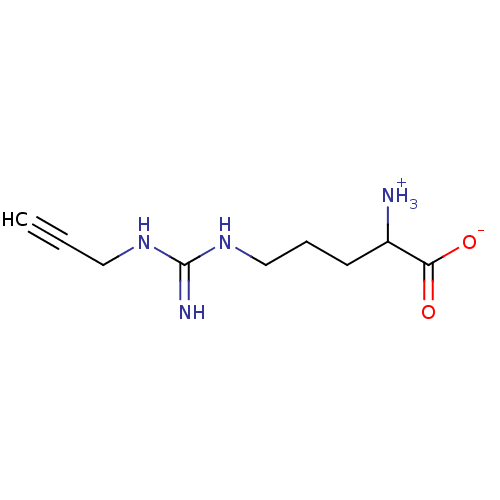
(2-Amino-5-(N'-prop-2-ynyl-guanidino)-pentanoic aci...)Show InChI InChI=1S/C9H16N4O2/c1-2-5-12-9(11)13-6-3-4-7(10)8(14)15/h1,7H,3-6,10H2,(H,14,15)(H3,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against recombinant inducible nitric oxide synthase (iNOS) from mouse |
J Med Chem 40: 3869-70 (1998)
Article DOI: 10.1021/jm970550g
BindingDB Entry DOI: 10.7270/Q2C829ZT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50330868
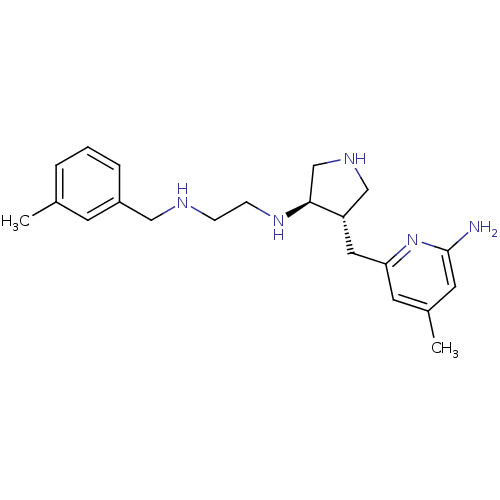
(CHEMBL1277509 | trans rac-N1-(4-((6-amino-4-methyl...)Show SMILES Cc1cccc(CNCCN[C@H]2CNC[C@@H]2Cc2cc(C)cc(N)n2)c1 |r| Show InChI InChI=1S/C21H31N5/c1-15-4-3-5-17(8-15)12-23-6-7-25-20-14-24-13-18(20)11-19-9-16(2)10-21(22)26-19/h3-5,8-10,18,20,23-25H,6-7,11-14H2,1-2H3,(H2,22,26)/t18-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50592473
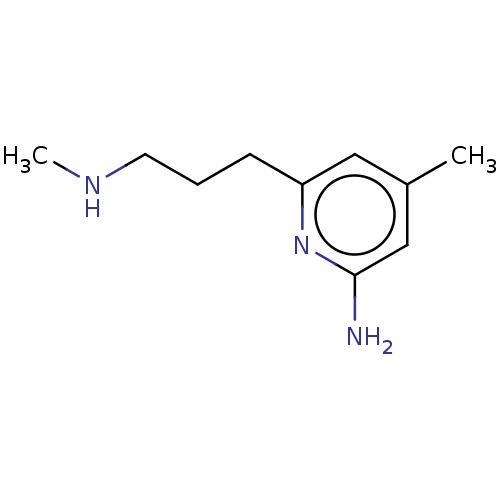
(CHEMBL5191295) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 669 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116878
BindingDB Entry DOI: 10.7270/Q2VQ36P6 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50225106

((2S)-2-amino-5-{[(E)-amino(nitroimino)methyl]amino...)Show SMILES N[C@@H](CCCNC(N)=N[N+]([O-])=O)C(O)=O |r,w:8.8| Show InChI InChI=1S/C6H13N5O4/c7-4(5(12)13)2-1-3-9-6(8)10-11(14)15/h4H,1-3,7H2,(H,12,13)(H3,8,9,10)/t4-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50341684
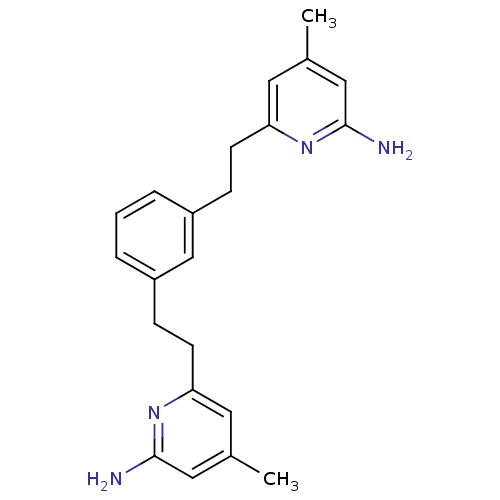
(6,6'-(2,2'-(1,3-Phenylene)bis(ethane-2,1-diyl))bis...)Show InChI InChI=1S/C22H26N4/c1-15-10-19(25-21(23)12-15)8-6-17-4-3-5-18(14-17)7-9-20-11-16(2)13-22(24)26-20/h3-5,10-14H,6-9H2,1-2H3,(H2,23,25)(H2,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| 682 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astex
| Assay Description
The NOSs isoform assays involved subjecting 3-8 to an oxyhemoglobin NO capture assay using a Biotek Gen5™ microplate reader. IC50 values for each com... |
J Med Chem 52: 379-88 (2009)
BindingDB Entry DOI: 10.7270/Q21N83G1 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50341684

(6,6'-(2,2'-(1,3-Phenylene)bis(ethane-2,1-diyl))bis...)Show InChI InChI=1S/C22H26N4/c1-15-10-19(25-21(23)12-15)8-6-17-4-3-5-18(14-17)7-9-20-11-16(2)13-22(24)26-20/h3-5,10-14H,6-9H2,1-2H3,(H2,23,25)(H2,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| 682 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
US Patent
| Assay Description
Enzyme inhibition was evaluated by measuring NO production with the hemoglobin capture assay, which was performed with purified NOSs in 96-well plate... |
US Patent US10759791 (2020)
BindingDB Entry DOI: 10.7270/Q2ZW1PZG |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50341684

(6,6'-(2,2'-(1,3-Phenylene)bis(ethane-2,1-diyl))bis...)Show InChI InChI=1S/C22H26N4/c1-15-10-19(25-21(23)12-15)8-6-17-4-3-5-18(14-17)7-9-20-11-16(2)13-22(24)26-20/h3-5,10-14H,6-9H2,1-2H3,(H2,23,25)(H2,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 682 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse macrophage recombinant iNOS expressed in Escherichia coli by UV-vis spectrometric analysis |
J Med Chem 54: 2039-48 (2011)
Article DOI: 10.1021/jm101071n
BindingDB Entry DOI: 10.7270/Q2571CB0 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50095177
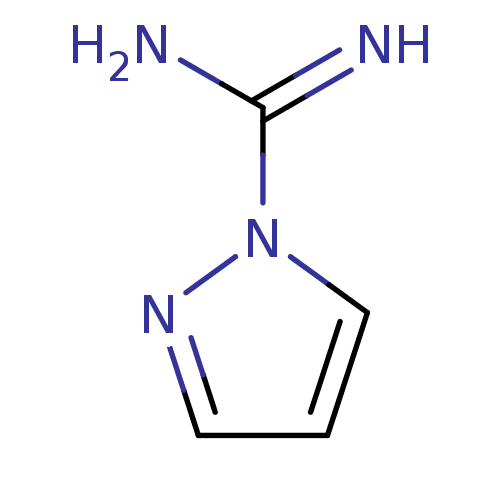
(CHEMBL503356 | CHEMBL542185 | Pyrazole-1-carboxami...)Show InChI InChI=1S/C4H6N4/c5-4(6)8-3-1-2-7-8/h1-3H,(H3,5,6) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition against induced Nitric Oxide Synthase(iNOS) |
Bioorg Med Chem Lett 10: 2771-4 (2000)
BindingDB Entry DOI: 10.7270/Q23B60NS |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50330867
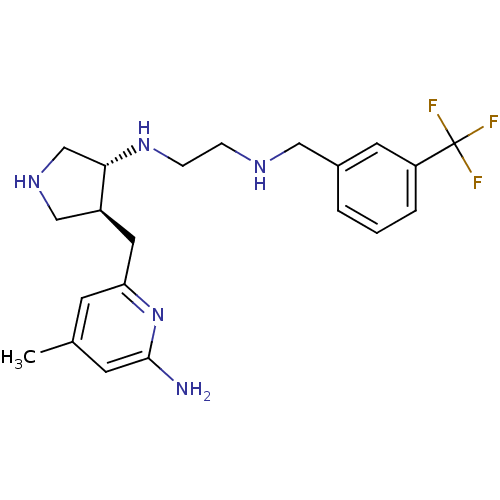
(CHEMBL1277508 | trans rac-N1-(4-((6-amino-4-methyl...)Show SMILES Cc1cc(N)nc(C[C@H]2CNC[C@@H]2NCCNCc2cccc(c2)C(F)(F)F)c1 |r| Show InChI InChI=1S/C21H28F3N5/c1-14-7-18(29-20(25)8-14)10-16-12-27-13-19(16)28-6-5-26-11-15-3-2-4-17(9-15)21(22,23)24/h2-4,7-9,16,19,26-28H,5-6,10-13H2,1H3,(H2,25,29)/t16-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Mus musculus (mouse)) | BDBM50330896
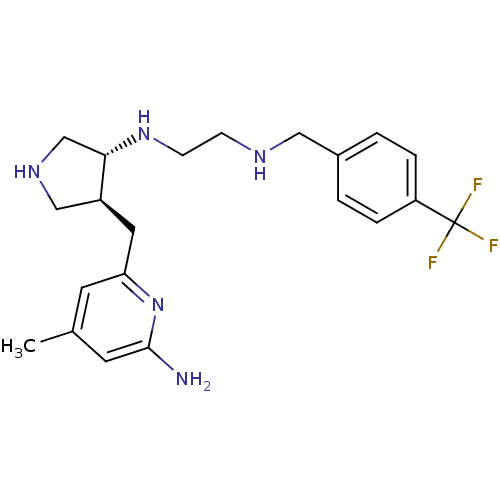
(CHEMBL1277419 | trans rac-N1-(4-((6-amino-4-methyl...)Show SMILES Cc1cc(N)nc(C[C@H]2CNC[C@@H]2NCCNCc2ccc(cc2)C(F)(F)F)c1 |r| Show InChI InChI=1S/C21H28F3N5/c1-14-8-18(29-20(25)9-14)10-16-12-27-13-19(16)28-7-6-26-11-15-2-4-17(5-3-15)21(22,23)24/h2-5,8-9,16,19,26-28H,6-7,10-13H2,1H3,(H2,25,29)/t16-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant iNOS |
J Med Chem 53: 7804-24 (2010)
Article DOI: 10.1021/jm100947x
BindingDB Entry DOI: 10.7270/Q2NC61FT |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM275051

(US9878996, Compound 7)Show InChI InChI=1S/C18H20FN5/c19-16-5-1-3-15(13-16)4-2-8-20-9-6-17-7-10-22-18(23-17)24-12-11-21-14-24/h1,3,5,7,10-14,20H,2,4,6,8-9H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| US Patent
| 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
US Patent
| Assay Description
Assays were performed at the NIMH Psychoactive Drug Screening Program (PDSP) at UNC-Chapel Hill. |
US Patent US9878996 (2018)
BindingDB Entry DOI: 10.7270/Q2PN97PN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data