

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ryanodine receptor 1 (Homo sapiens (Human)) | BDBM50051434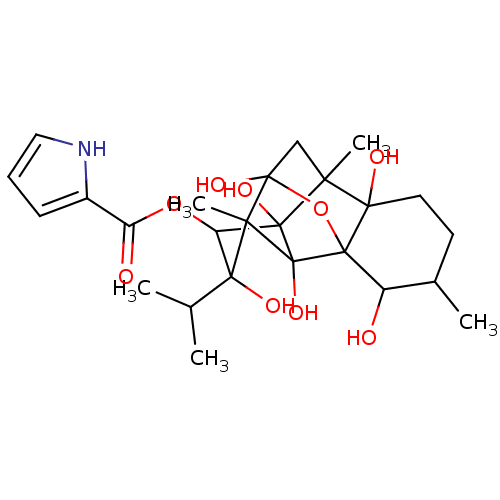 (10-epi-hydroxy-Ryanodine | CHEMBL308183 | Ryanodin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Binding to ryanodine receptor in canine cardiac membranes. | J Med Chem 39: 2331-8 (1996) Article DOI: 10.1021/jm950711l BindingDB Entry DOI: 10.7270/Q24Q7T31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430056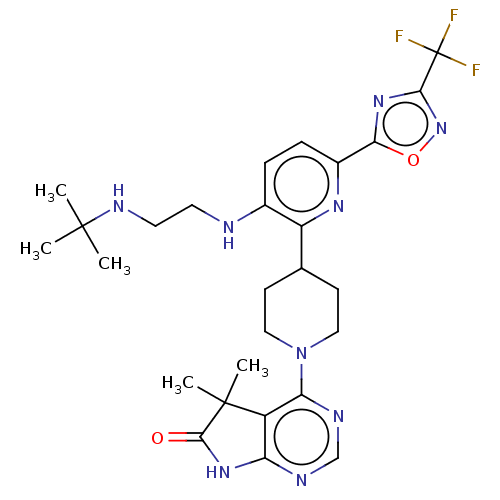 (US10538528, Compound 43) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430057 (US10538528, Compound 44) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430051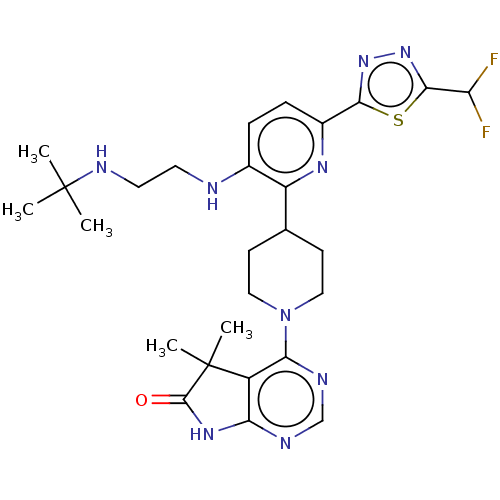 (US10538528, Compound 37) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430052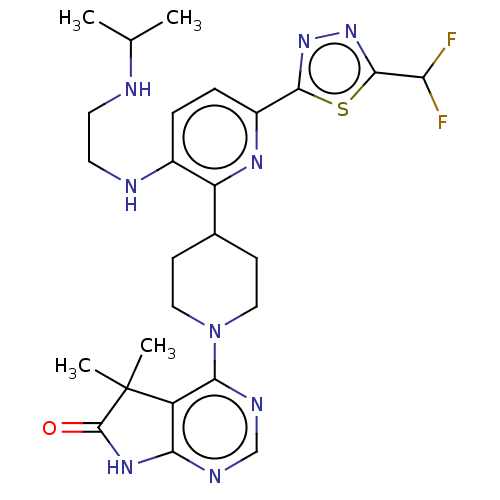 (US10538528, Compound 39) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430053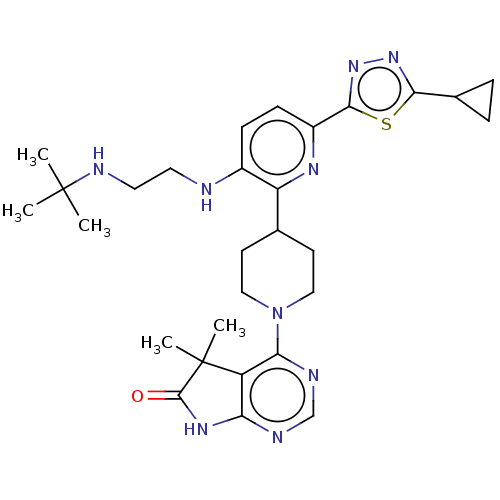 (US10538528, Compound 40) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430047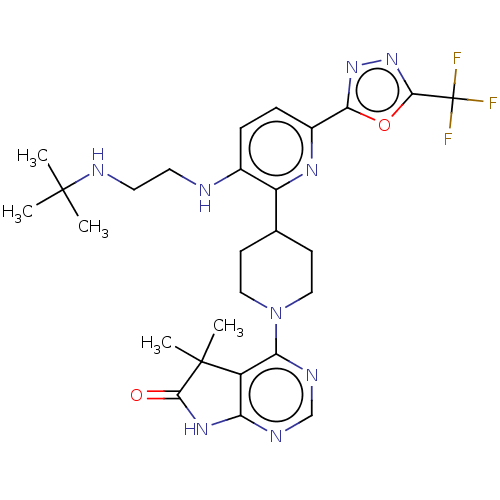 (US10538528, Compound 32) | UniProtKB/SwissProt GoogleScholar AffyNet | MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430030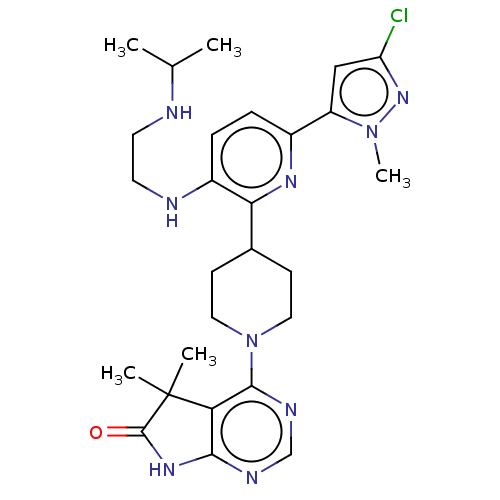 (US10538528, Compound 11) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430029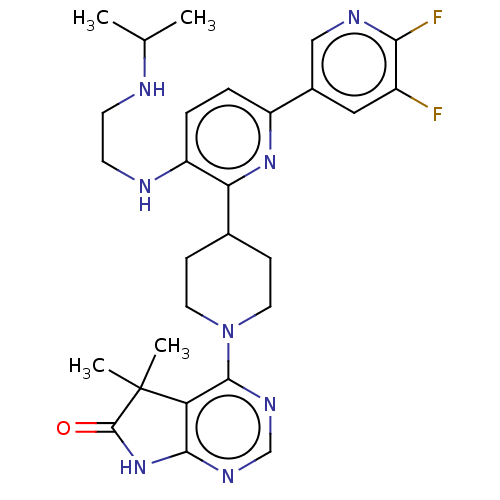 (US10538528, Compound 5) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430034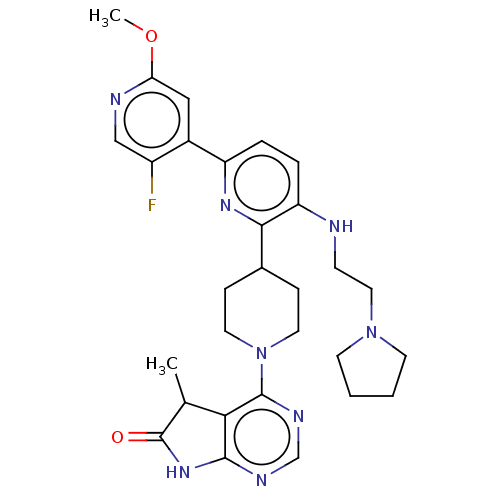 (US10538528, Compound 16) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430050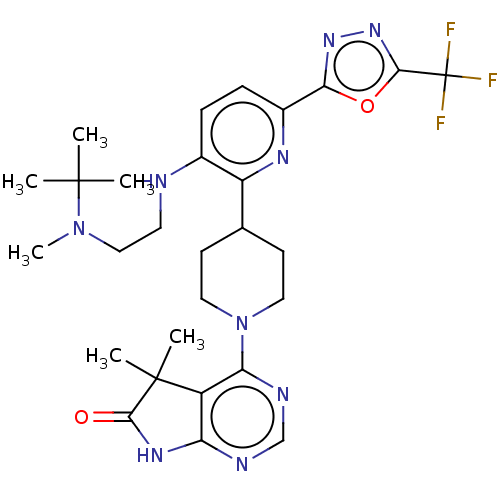 (US10538528, Compound 34) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430038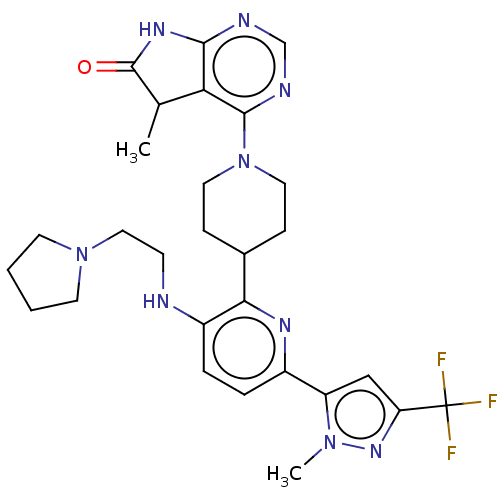 (US10538528, Compound 20) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430066 (US10538528, Compound 38) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430037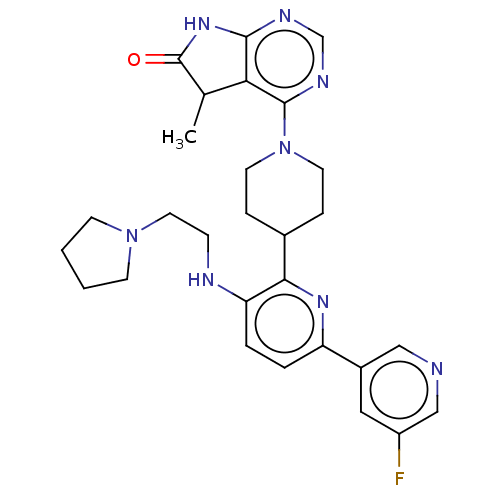 (US10538528, Compound 19) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430063 (US10538528, Compound 10) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430049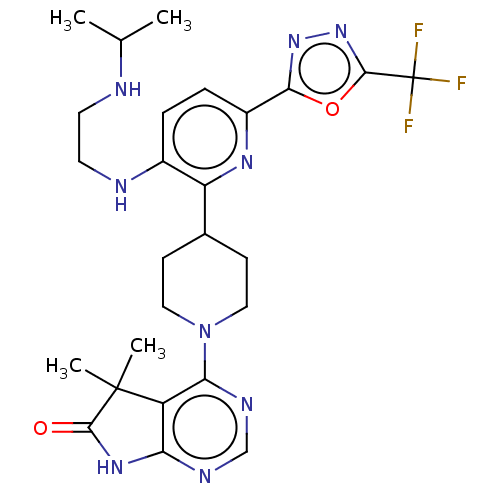 (US10538528, Compound 33) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430059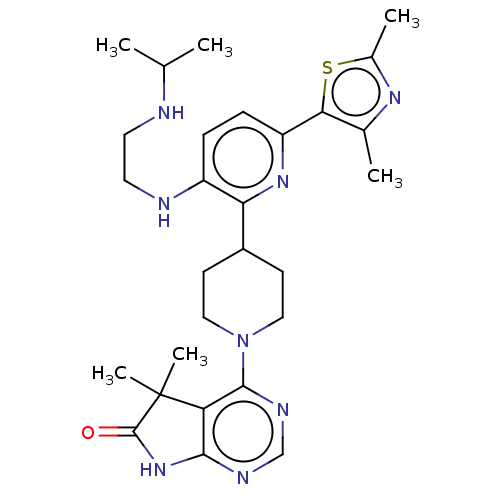 (US10538528, Compound 6) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430035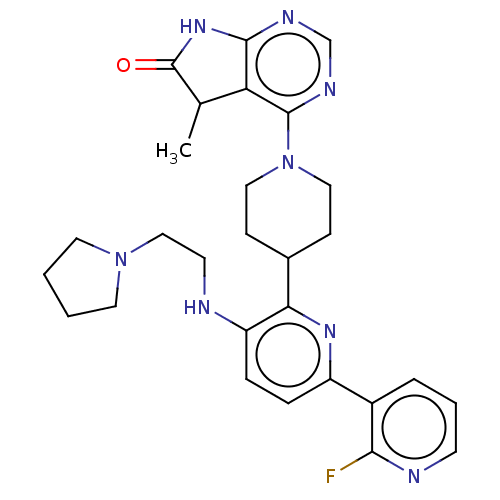 (US10538528, Compound 17) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430033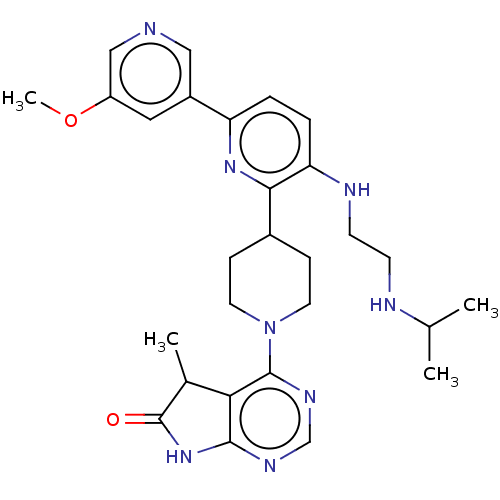 (US10538528, Compound 15) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430062 (US10538528, Compound 9) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 (Oryctolagus cuniculus) | BDBM50044989 ((O10eq)-beta-guanidinopropionylryanodine | CHEMBL2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Curated by ChEMBL | Assay Description Affinity for sarcoplasmic reticular calcium release channel (SR CRC) of rabbit skeletal membrane vesicles | J Med Chem 36: 1319-23 (1993) BindingDB Entry DOI: 10.7270/Q2PN968V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430055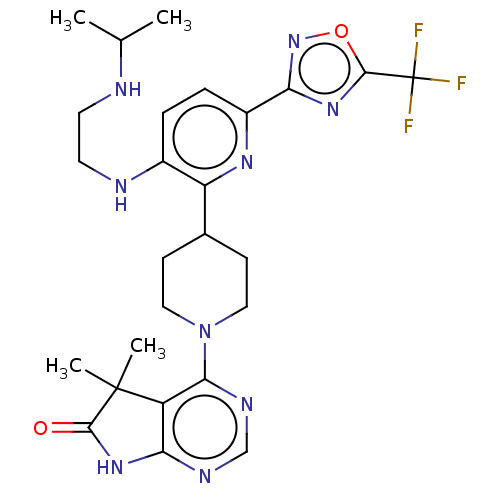 (US10538528, Compound 42) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430039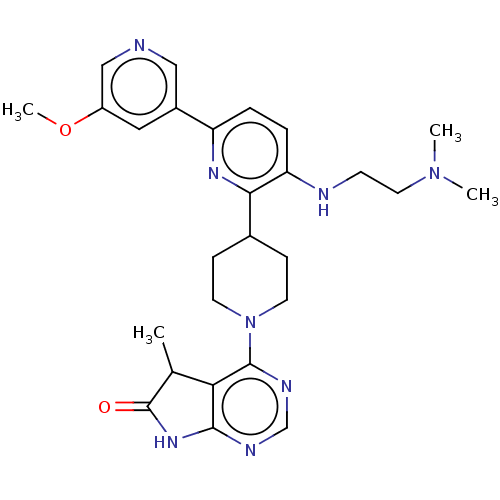 (US10538528, Compound 21) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430036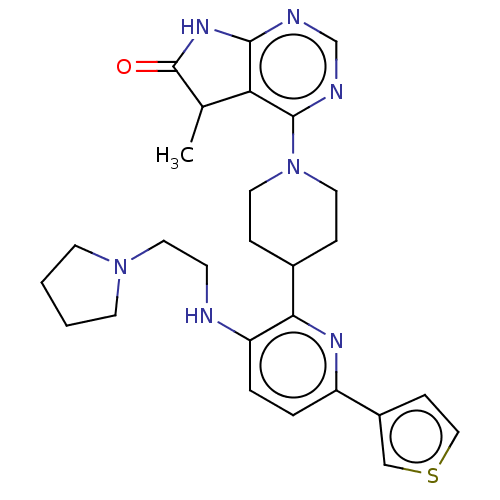 (US10538528, Compound 18) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430031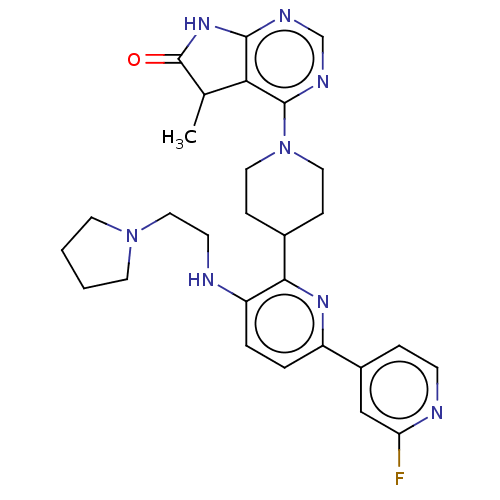 (US10538528, Compound 13) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430041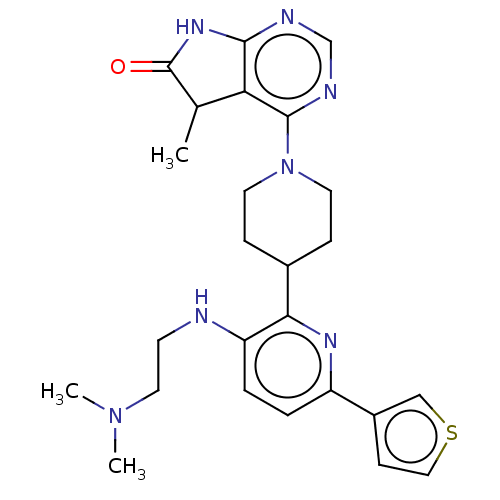 (US10538528, Compound 23) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430058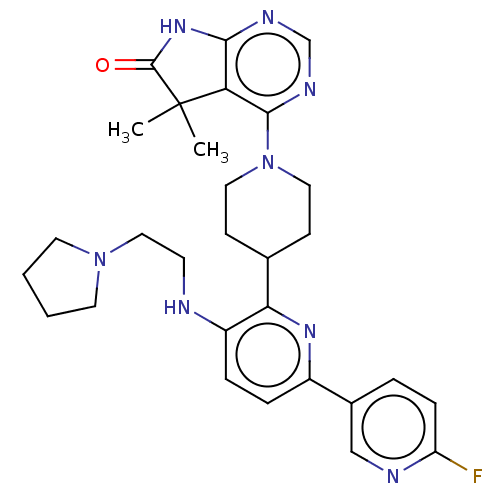 (US10538528, Compound 2) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430028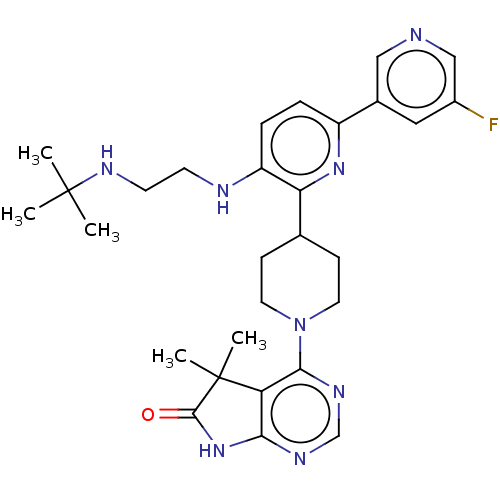 (US10538528, Compound 1) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430065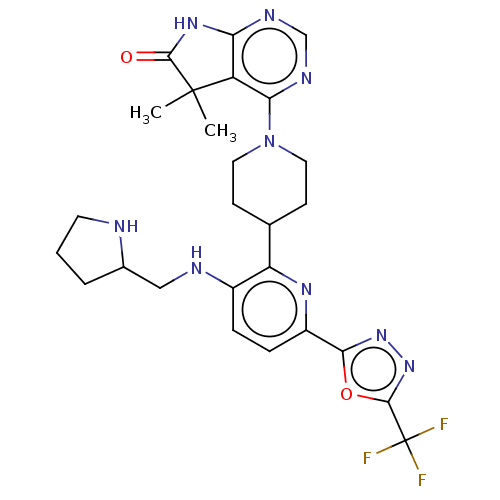 (US10538528, Compound 35) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430054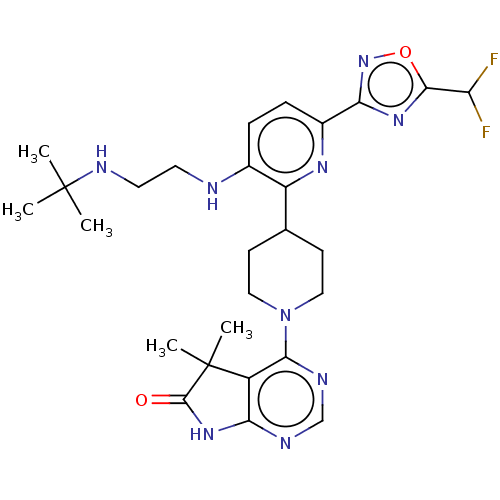 (US10538528, Compound 41) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430060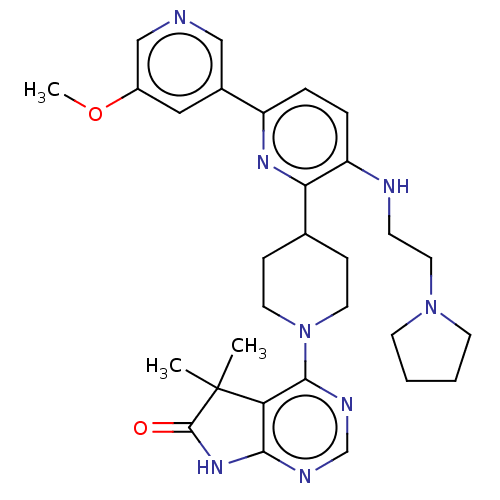 (US10538528, Compound 7) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430032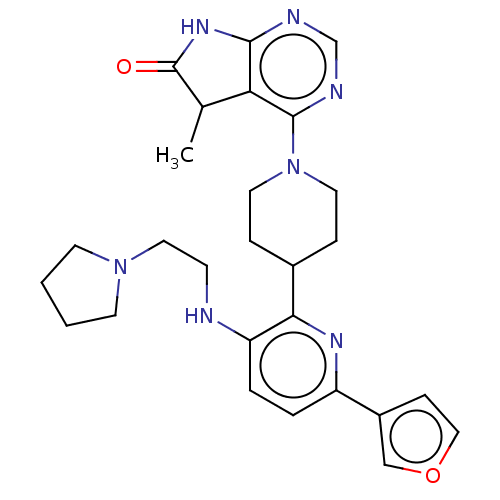 (US10538528, Compound 14) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430045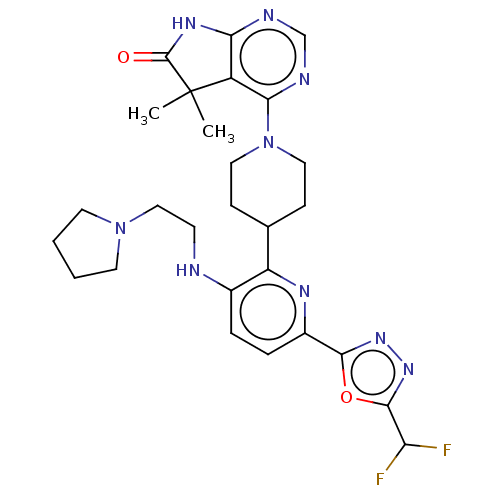 (US10538528, Compound 30) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430061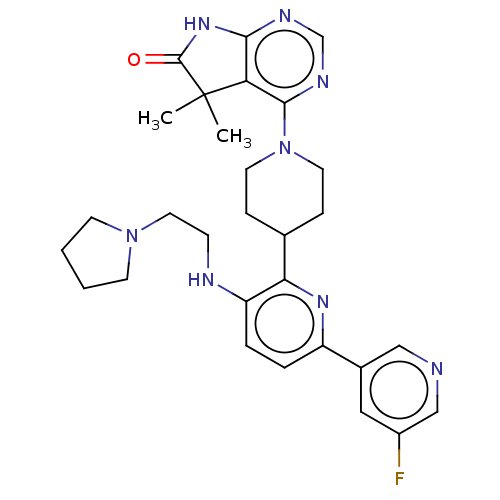 (US10538528, Compound 8) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 (Oryctolagus cuniculus) | BDBM50044982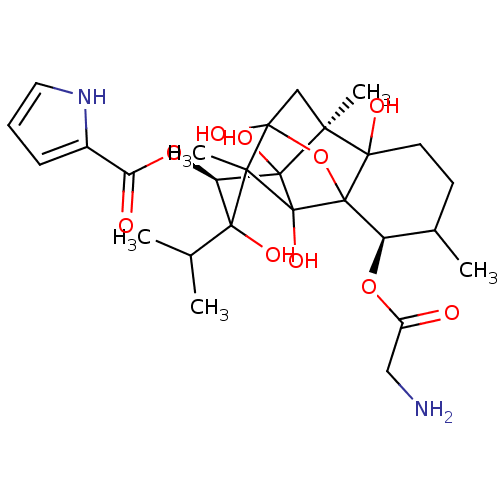 (CHEMBL283836 | GLYCYLRYANODINE) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Curated by ChEMBL | Assay Description Affinity for sarcoplasmic reticular calcium release channel (SR CRC) of rabbit skeletal membrane vesicles | J Med Chem 36: 1319-23 (1993) BindingDB Entry DOI: 10.7270/Q2PN968V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 (Oryctolagus cuniculus) | BDBM50044986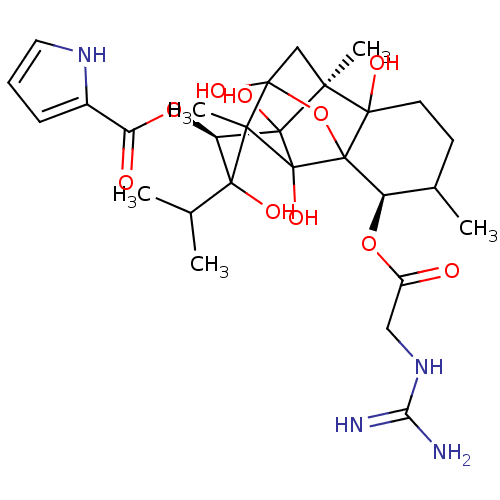 ((O10eq)-guanidino acetylryanodine | CHEMBL435362) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Curated by ChEMBL | Assay Description Affinity for sarcoplasmic reticular calcium release channel (SR CRC) of rabbit skeletal membrane vesicles | J Med Chem 36: 1319-23 (1993) BindingDB Entry DOI: 10.7270/Q2PN968V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430064 (US10538528, Compound 12) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430044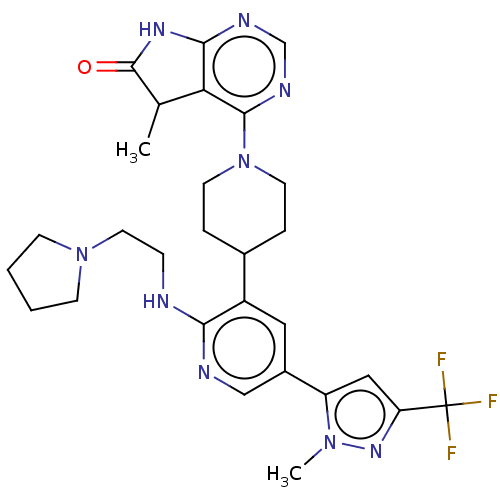 (US10538528, Compound 27) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 [4317-4329] () | BDBM430046 (US10538528, Compound 31) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pahrmaceutical Co., Ltd. US Patent | Assay Description Rsk1: Inhibitory activity of the compound according to the present invention on Rsk1 kinase activity in vitro was assayed using the QSS Assist FP ass... | US Patent US10538528 (2020) BindingDB Entry DOI: 10.7270/Q2MK6G95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 (Oryctolagus cuniculus) | BDBM50044994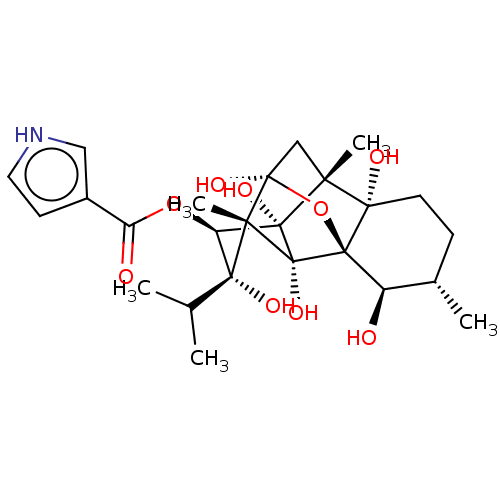 (2,6,9,11,13,14-hexahydroxy-11-isopropyl-3,7,10-tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Displacement of [3H]ryanodine from Ca2+-ryanodine receptor in Oryctolagus cuniculus (rabbit) skeletal muscle sarcoplasmic reticulum after 2 hr by com... | J Med Chem 36: 1128-35 (1993) Article DOI: 10.1021/jm00061a003 BindingDB Entry DOI: 10.7270/Q2JS9TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 (Oryctolagus cuniculus) | BDBM50044995 ((O10eq)-beta-alanylryanodine | CHEMBL282089) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Curated by ChEMBL | Assay Description Affinity for sarcoplasmic reticular calcium release channel (SR CRC) of rabbit skeletal membrane vesicles | J Med Chem 36: 1319-23 (1993) BindingDB Entry DOI: 10.7270/Q2PN968V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 (Homo sapiens (Human)) | BDBM50051434 (10-epi-hydroxy-Ryanodine | CHEMBL308183 | Ryanodin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against ryanodine receptor from mouse brain preparation | J Med Chem 39: 2331-8 (1996) Article DOI: 10.1021/jm950711l BindingDB Entry DOI: 10.7270/Q24Q7T31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 (Oryctolagus cuniculus) | BDBM50044994 (2,6,9,11,13,14-hexahydroxy-11-isopropyl-3,7,10-tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Displacement of [3H]ryanodine from Ca2+-ryanodine receptor in Oryctolagus cuniculus (rabbit) skeletal muscle sarcoplasmic reticulum after 1 hr by com... | J Med Chem 36: 1128-35 (1993) Article DOI: 10.1021/jm00061a003 BindingDB Entry DOI: 10.7270/Q2JS9TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 (Homo sapiens (Human)) | BDBM50051434 (10-epi-hydroxy-Ryanodine | CHEMBL308183 | Ryanodin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibitory activity against ryanodine receptor from rabbit muscle preparation | J Med Chem 39: 2331-8 (1996) Article DOI: 10.1021/jm950711l BindingDB Entry DOI: 10.7270/Q24Q7T31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 (Oryctolagus cuniculus) | BDBM50044993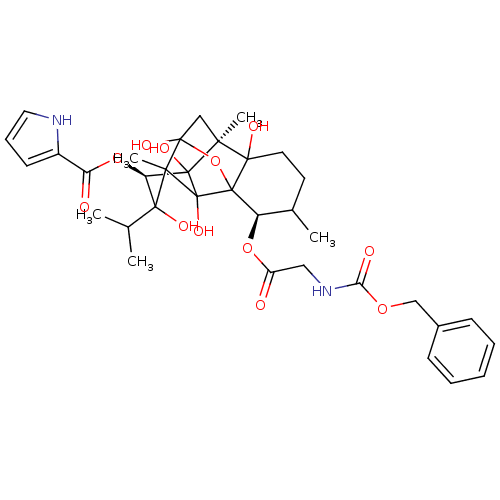 (Benzyloxycarbonyl-glycylryanodine | CHEMBL28383) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Curated by ChEMBL | Assay Description Affinity for sarcoplasmic reticular calcium release channel (SR CRC) of rabbit skeletal membrane vesicles | J Med Chem 36: 1319-23 (1993) BindingDB Entry DOI: 10.7270/Q2PN968V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 (Oryctolagus cuniculus) | BDBM50044990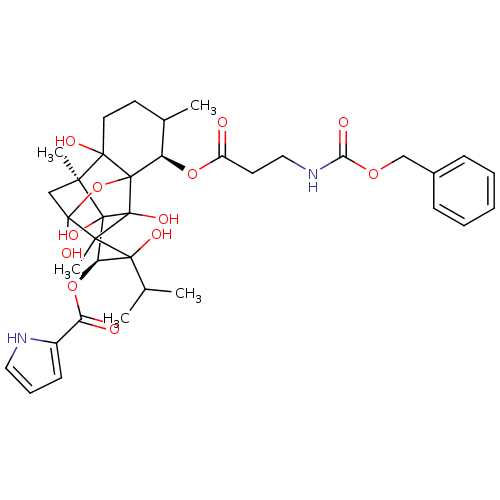 ((O10eq)-Benzyloxycarbonyl-beta-alanylryanodine | C...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Curated by ChEMBL | Assay Description Affinity for sarcoplasmic reticular calcium release channel (SR CRC) of rabbit skeletal membrane vesicles | J Med Chem 36: 1319-23 (1993) BindingDB Entry DOI: 10.7270/Q2PN968V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 (Oryctolagus cuniculus) | BDBM50044994 (2,6,9,11,13,14-hexahydroxy-11-isopropyl-3,7,10-tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Curated by ChEMBL | Assay Description Affinity for sarcoplasmic reticular calcium release channel (SR CRC) of rabbit skeletal membrane vesicles | J Med Chem 36: 1319-23 (1993) BindingDB Entry DOI: 10.7270/Q2PN968V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 (Oryctolagus cuniculus) | BDBM50044991 (2,6,9,11,13,14-hexahydroxy-11-isopropyl-7,10-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University Curated by ChEMBL | Assay Description Affinity for sarcoplasmic reticular calcium release channel (SR CRC) of rabbit skeletal membrane vesicles | J Med Chem 36: 1319-23 (1993) BindingDB Entry DOI: 10.7270/Q2PN968V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 (Oryctolagus cuniculus) | BDBM50044994 (2,6,9,11,13,14-hexahydroxy-11-isopropyl-3,7,10-tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]ryanodine from Ca2+-ryanodine receptor in Oryctolagus cuniculus (rabbit) skeletal muscle sarcoplasmic reticulum after 80 min by c... | J Med Chem 30: 710-6 (1987) BindingDB Entry DOI: 10.7270/Q26M37D3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ryanodine receptor 1 (Oryctolagus cuniculus) | BDBM50016403 (2,6,9,11,13,14-hexahydroxy-11-isopropyl-7,10-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]ryanodine from Ca2+-ryanodine receptor in Oryctolagus cuniculus (rabbit) skeletal muscle sarcoplasmic reticulum after 80 min by c... | J Med Chem 30: 710-6 (1987) BindingDB Entry DOI: 10.7270/Q26M37D3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 89 total ) | Next | Last >> |