

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50442489 (CHEMBL2440417) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Competitive inhibition of wild-type human recombinant N-terminal His6-tagged AKR1B10 expressed in Escherichia coli using geraniol as substrate by dou... | Bioorg Med Chem 21: 6378-84 (2013) Article DOI: 10.1016/j.bmc.2013.08.059 BindingDB Entry DOI: 10.7270/Q25T3MX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50241817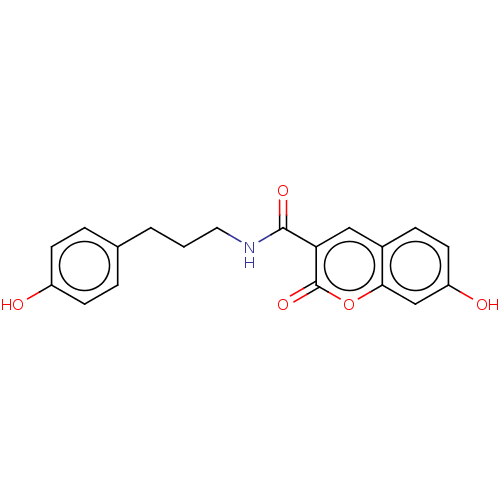 (CHEMBL4081954) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Competitive inhibition of recombinant human AKR1B10 in presence of geraniol as substrate by Lineweaver-Burk plot method | J Med Chem 60: 8441-8455 (2017) Article DOI: 10.1021/acs.jmedchem.7b00830 BindingDB Entry DOI: 10.7270/Q2NZ89S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50362835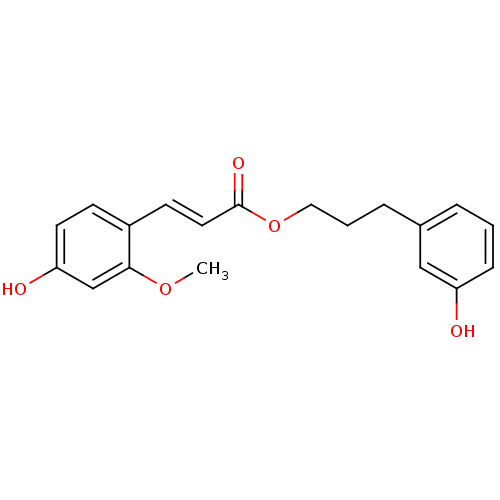 (CHEMBL1940400) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Competitive inhibition at human recombinant N-terminus His6-tagged AKR1B10 expressed in Escherichia coli BL21 DE3 assessed as inhibition of NADP+ lin... | Eur J Med Chem 48: 321-9 (2012) Article DOI: 10.1016/j.ejmech.2011.12.034 BindingDB Entry DOI: 10.7270/Q2TT4RDB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50321717 (7-hydroxy-2-(4-methoxyphenylimino)-N-(pyridin-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of dehydrogenase activity of N-terminal 6His-tagged AKR1B10 expressed in Escherichia coli BL21(DE3) assessed as inhibition of geraniol deh... | Bioorg Med Chem 18: 2485-90 (2010) Article DOI: 10.1016/j.bmc.2010.02.050 BindingDB Entry DOI: 10.7270/Q2K35TTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50506669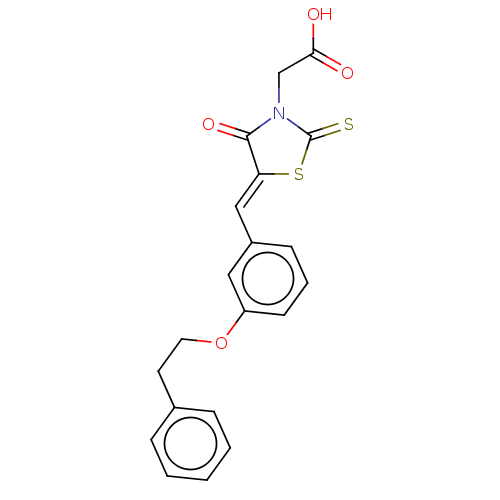 (CHEMBL4459307) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Uncompetitive inhibition of human recombinant aldose reductase expressed in Escherichia coli BL21(DE3)pLysS assessed as dissociation constant of enzy... | Bioorg Med Chem Lett 28: 3712-3720 (2018) Article DOI: 10.1016/j.bmcl.2018.10.024 BindingDB Entry DOI: 10.7270/Q2QJ7MKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50321718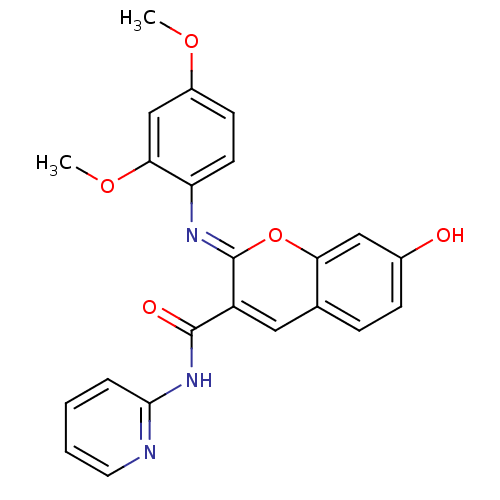 (2-(2,4-dimethoxyphenylimino)-7-hydroxy-N-(pyridin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of dehydrogenase activity of N-terminal 6His-tagged AKR1B10 expressed in Escherichia coli BL21(DE3) assessed as inhibition of geraniol deh... | Bioorg Med Chem 18: 2485-90 (2010) Article DOI: 10.1016/j.bmc.2010.02.050 BindingDB Entry DOI: 10.7270/Q2K35TTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50394657 (CHEMBL270067) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of reductase activity of N-terminal 6His-tagged human AKR1B10 expressed in Escherichia coli BL21(DE3) assessed as pyridine-3-aldehyde redu... | J Nat Prod 74: 1201-6 (2011) Article DOI: 10.1021/np200118q BindingDB Entry DOI: 10.7270/Q25D8SXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50321715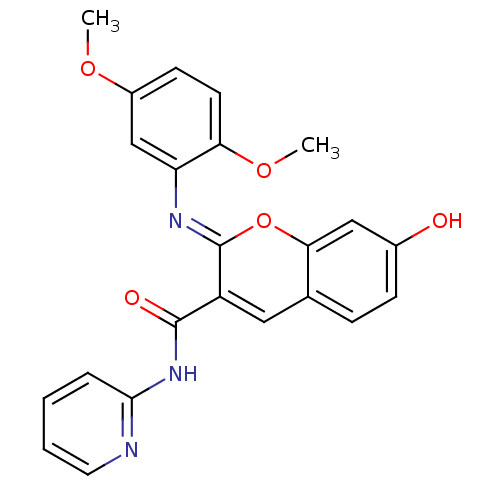 (2-(2,5-dimethoxyphenylimino)-7-hydroxy-N-(pyridin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of dehydrogenase activity of N-terminal 6His-tagged AKR1B10 expressed in Escherichia coli BL21(DE3) assessed as inhibition of geraniol deh... | Bioorg Med Chem 18: 2485-90 (2010) Article DOI: 10.1016/j.bmc.2010.02.050 BindingDB Entry DOI: 10.7270/Q2K35TTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50506667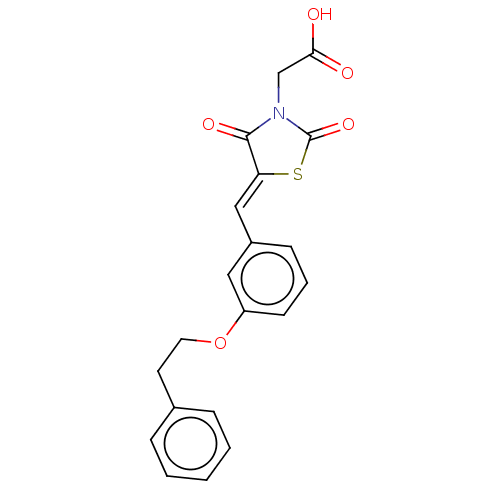 (CHEMBL4448571) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Uncompetitive inhibition of human recombinant aldose reductase expressed in Escherichia coli BL21(DE3)pLysS assessed as dissociation constant of enzy... | Bioorg Med Chem Lett 28: 3712-3720 (2018) Article DOI: 10.1016/j.bmcl.2018.10.024 BindingDB Entry DOI: 10.7270/Q2QJ7MKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Rattus norvegicus) | BDBM50451729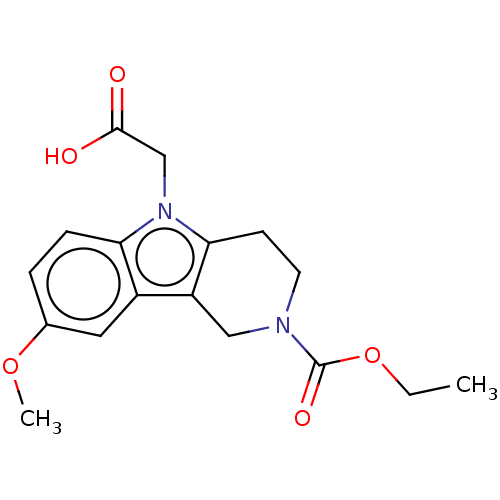 (CHEMBL4206601) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Slovak Academy of Sciences Curated by ChEMBL | Assay Description Uncompetitive inhibition of ALR2 in Wistar rat eye lens assessed as reduction in NADPH consumption preincubated for 1 min followed by D,L-glyceraldeh... | Bioorg Med Chem 25: 6353-6360 (2017) Article DOI: 10.1016/j.bmc.2017.10.005 BindingDB Entry DOI: 10.7270/Q23T9KSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16452 ((4-oxo-3-{[5-(trifluoromethyl)-1,3-benzothiazol-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of aldose reductase (aldo-keto reductase, AKR1B1) isolated from human placenta. | J Med Chem 34: 108-22 (1991) BindingDB Entry DOI: 10.7270/Q2513X6H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50321716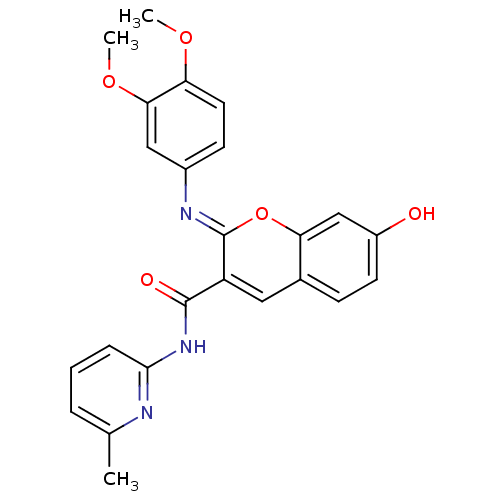 (2-(3,4-dimethoxyphenylimino)-7-hydroxy-N-(6-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of dehydrogenase activity of N-terminal 6His-tagged AKR1B10 expressed in Escherichia coli BL21(DE3) assessed as inhibition of geraniol deh... | Bioorg Med Chem 18: 2485-90 (2010) Article DOI: 10.1016/j.bmc.2010.02.050 BindingDB Entry DOI: 10.7270/Q2K35TTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50029207 ((E)-3-(3,4-Dihydroxy-phenyl)-acrylic acid phenethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Mixed-type inhibition at human recombinant N-terminus His6-tagged AKR1B10 expressed in Escherichia coli BL21 DE3 assessed as inhibition of NADP+ link... | Eur J Med Chem 48: 321-9 (2012) Article DOI: 10.1016/j.ejmech.2011.12.034 BindingDB Entry DOI: 10.7270/Q2TT4RDB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM16314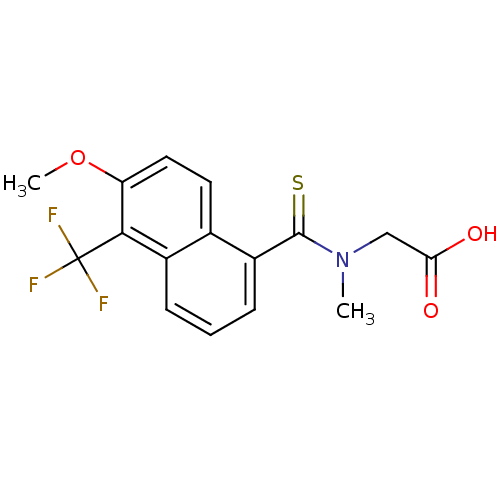 (2-{[6-methoxy-5-(trifluoromethyl)naphthalen-1-yl]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of reductase activity of N-terminal 6His-tagged human AKR1B10 expressed in Escherichia coli BL21(DE3) assessed as pyridine-3-aldehyde redu... | J Nat Prod 74: 1201-6 (2011) Article DOI: 10.1021/np200118q BindingDB Entry DOI: 10.7270/Q25D8SXZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50451729 (CHEMBL4206601) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Slovak Academy of Sciences Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant human AKR1B1 using D,L-glyceraldehyde as substrate | Bioorg Med Chem 25: 6353-6360 (2017) Article DOI: 10.1016/j.bmc.2017.10.005 BindingDB Entry DOI: 10.7270/Q23T9KSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50346601 (NSC-114945 | OLEANOLIC_ACID | Oleanolic acid | Ole...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Competitive inhibition of dehydrogenase activity of N-terminal 6His-tagged human AKR1B10 expressed in Escherichia coli BL21(DE3) assessed as NADP+-li... | J Nat Prod 74: 1201-6 (2011) Article DOI: 10.1021/np200118q BindingDB Entry DOI: 10.7270/Q25D8SXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Rattus norvegicus) | BDBM50080464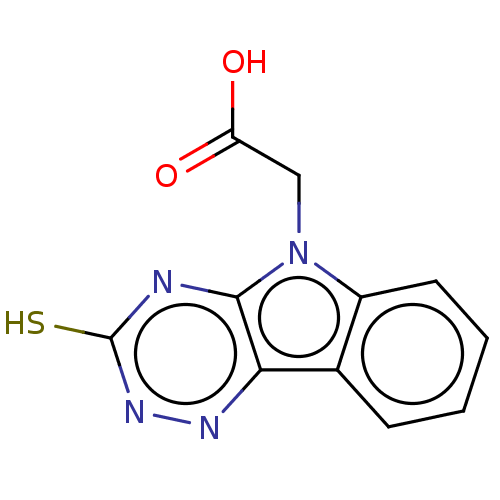 (ALR2 inhibitor, 12 | CHEMBL1405739) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Slovak Academy of Sciences Curated by ChEMBL | Assay Description Uncompetitive inhibition of Wistar rat lens aldose reductase using D,L-glyceraldehyde as substrate by double reciprocal plot analysis | J Med Chem 58: 2649-57 (2015) Article DOI: 10.1021/jm5015814 BindingDB Entry DOI: 10.7270/Q2WS8VZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | CHEMBL5288197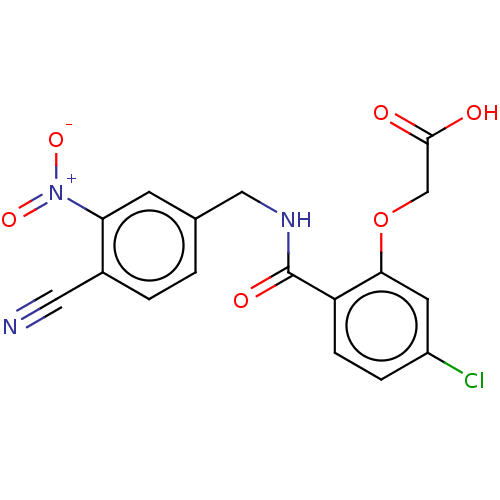 | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50010449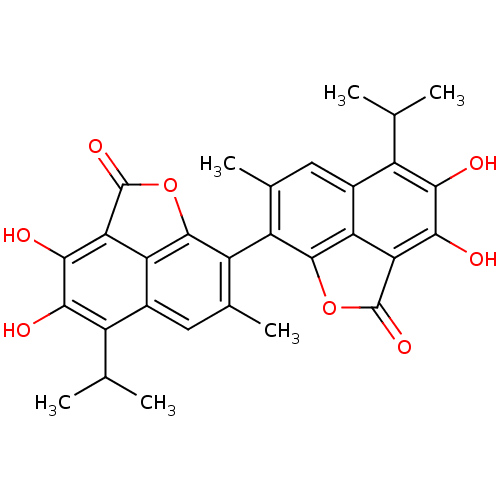 (3,4,3',4'-Tetrahydroxy-5,5'-diisopropyl-7,7'-dimet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against aldose reductase from human placenta | J Med Chem 34: 3301-5 (1991) BindingDB Entry DOI: 10.7270/Q2VM4B7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50506669 (CHEMBL4459307) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Uncompetitive inhibition of human recombinant aldose reductase expressed in Escherichia coli BL21(DE3)pLysS assessed as dissociation constant of enzy... | Bioorg Med Chem Lett 28: 3712-3720 (2018) Article DOI: 10.1016/j.bmcl.2018.10.024 BindingDB Entry DOI: 10.7270/Q2QJ7MKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50010436 (6,7,6',7'-Tetrahydroxy-5,5'-diisopropyl-1,1'-dimet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against aldose reductase from human placenta | J Med Chem 34: 3301-5 (1991) BindingDB Entry DOI: 10.7270/Q2VM4B7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50028365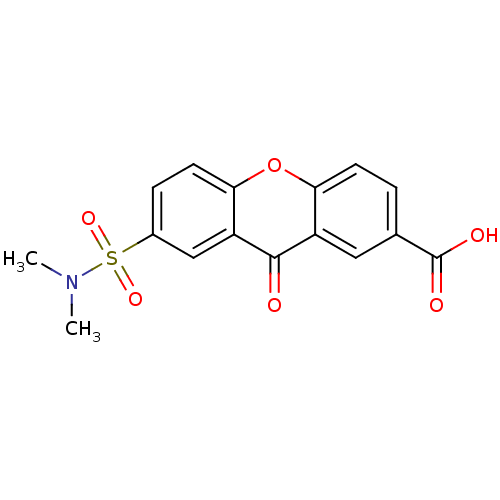 (7-Dimethylsulfamoyl-9-oxo-9H-xanthene-2-carboxylic...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity of the compound was determined towards rabbit aldose reductase | J Med Chem 23: 1264-7 (1981) BindingDB Entry DOI: 10.7270/Q2W95874 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM23223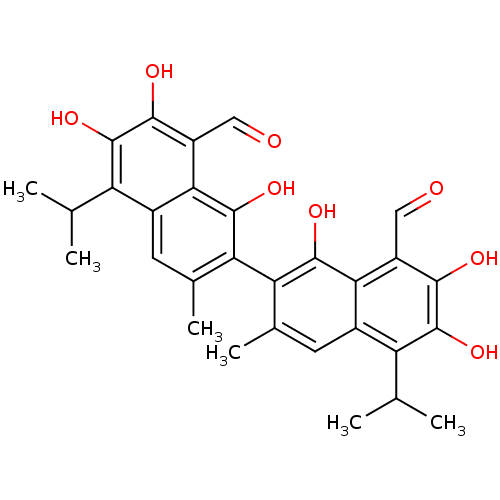 (7-[8-formyl-1,6,7-trihydroxy-3-methyl-5-(propan-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against Aldose reductase from human placenta | J Med Chem 34: 3301-5 (1991) BindingDB Entry DOI: 10.7270/Q2VM4B7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50506667 (CHEMBL4448571) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Uncompetitive inhibition of human recombinant aldose reductase expressed in Escherichia coli BL21(DE3)pLysS assessed as dissociation constant of enzy... | Bioorg Med Chem Lett 28: 3712-3720 (2018) Article DOI: 10.1016/j.bmcl.2018.10.024 BindingDB Entry DOI: 10.7270/Q2QJ7MKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16436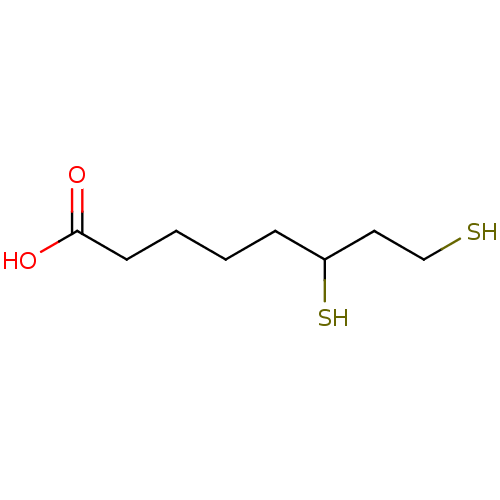 (6,8-disulfanyloctanoic acid | D,L-Dihydrolipoic ac...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 950 | -34.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Medical College of Wiscosin | Assay Description The production of NADPH from NADP+ and benzyl alcohol and xylitol was monitored by an increase in NADPH fluorescence (ex: 340 nm; em: 460 nm) using a... | Bioorg Chem 34: 424-44 (2006) Article DOI: 10.1016/j.bioorg.2006.09.004 BindingDB Entry DOI: 10.7270/Q2SN076W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 [W220Y,C299A] (Homo sapiens (Human)) | BDBM16429 (2,6-Dichlorophenylacetic acid | 2-(2,6-dichlorophe...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wiscosin | Assay Description The production of NADPH from NADP+ and benzyl alcohol and xylitol was monitored by an increase in NADPH fluorescence (ex: 340 nm; em: 460 nm) using a... | Bioorg Chem 34: 424-44 (2006) Article DOI: 10.1016/j.bioorg.2006.09.004 BindingDB Entry DOI: 10.7270/Q2SN076W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50010451 (But-2-enoic acid 1'-but-2-enoyloxy-8,8'-dicyano-6,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against Aldose reductase from human placenta | J Med Chem 34: 3301-5 (1991) BindingDB Entry DOI: 10.7270/Q2VM4B7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16417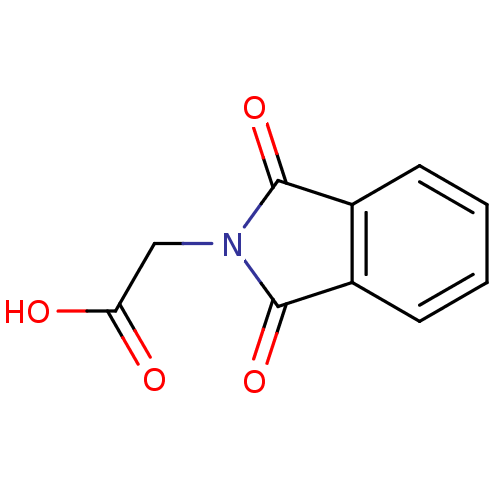 ((1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)acetic aci...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.50E+3 | -33.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Medical College of Wiscosin | Assay Description The production of NADPH from NADP+ and benzyl alcohol and xylitol was monitored by an increase in NADPH fluorescence (ex: 340 nm; em: 460 nm) using a... | Bioorg Chem 34: 424-44 (2006) Article DOI: 10.1016/j.bioorg.2006.09.004 BindingDB Entry DOI: 10.7270/Q2SN076W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50010446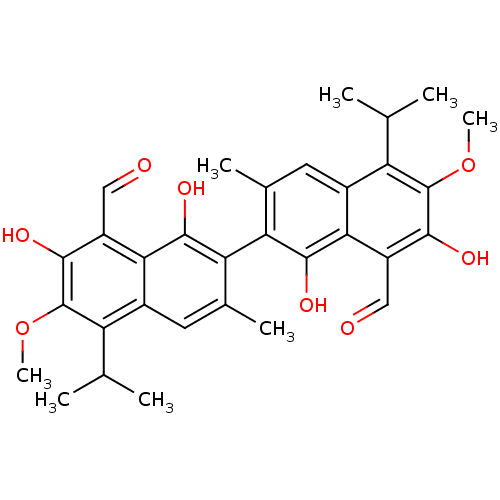 (1,7,1',7'-Tetrahydroxy-5,5'-diisopropyl-6,6'-dimet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against Aldose reductase from human placenta | J Med Chem 34: 3301-5 (1991) BindingDB Entry DOI: 10.7270/Q2VM4B7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | CHEMBL5280965 | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM50148911 ((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Competitive inhibition of dehydrogenase activity of N-terminal 6His-tagged human AKR1B10 expressed in Escherichia coli BL21(DE3) assessed as NADP+-li... | J Nat Prod 74: 1201-6 (2011) Article DOI: 10.1021/np200118q BindingDB Entry DOI: 10.7270/Q25D8SXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16415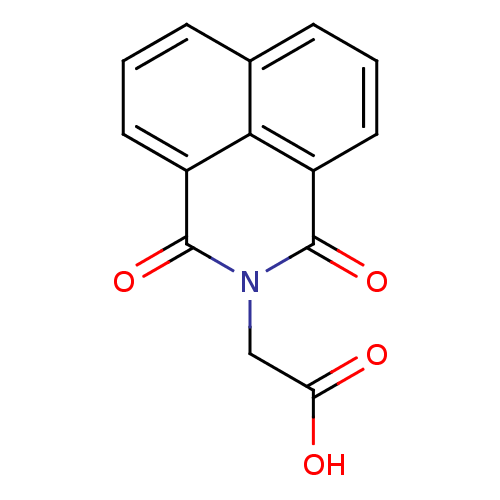 ((1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetic...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.00E+3 | -32.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Medical College of Wiscosin | Assay Description The production of NADPH from NADP+ and benzyl alcohol and xylitol was monitored by an increase in NADPH fluorescence (ex: 340 nm; em: 460 nm) using a... | Bioorg Chem 34: 424-44 (2006) Article DOI: 10.1016/j.bioorg.2006.09.004 BindingDB Entry DOI: 10.7270/Q2SN076W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM11318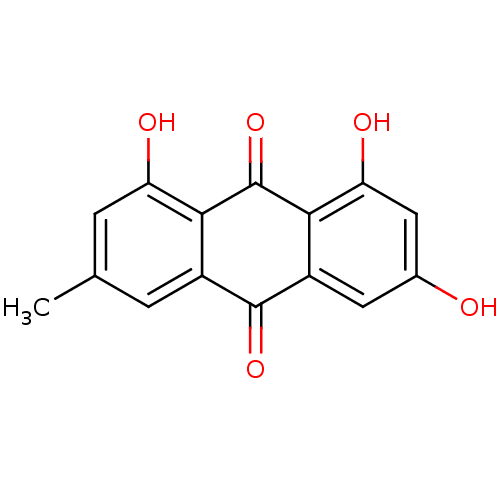 (1,3,8-trihydroxy-6-methyl-9,10-dihydroanthracene-9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Uncompetitive inhibition of human recombinant aldose reductase expressed in Escherichia coli using DL-glyceraldehyde as substrate by double reciproca... | J Nat Prod 79: 1439-44 (2016) Article DOI: 10.1021/acs.jnatprod.6b00185 BindingDB Entry DOI: 10.7270/Q2CN75T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM50010332 (CHEMBL3251376) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of beef brain NADP-dependent aldehyde reductase using p-nitrobenzaldehyde by competitive inhibition assay | J Med Chem 22: 1011-4 (1979) BindingDB Entry DOI: 10.7270/Q21C1ZD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16439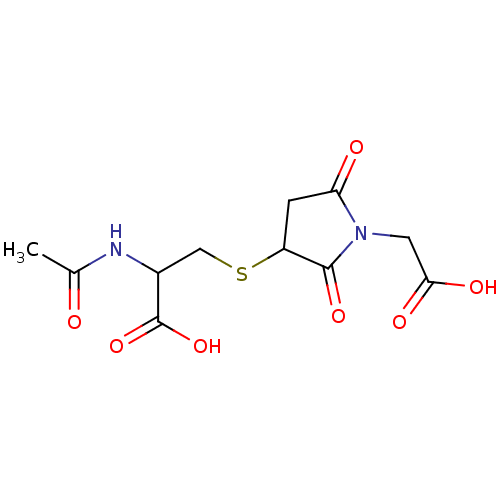 (3-{[1-(carboxymethyl)-2,5-dioxopyrrolidin-3-yl]sul...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wiscosin | Assay Description The production of NADPH from NADP+ and benzyl alcohol and xylitol was monitored by an increase in NADPH fluorescence (ex: 340 nm; em: 460 nm) using a... | Bioorg Chem 34: 424-44 (2006) Article DOI: 10.1016/j.bioorg.2006.09.004 BindingDB Entry DOI: 10.7270/Q2SN076W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16428 (2,6-Difluorophenylacetic acid | 2-(2,6-difluorophe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.50E+3 | -32.0 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Medical College of Wiscosin | Assay Description The production of NADPH from NADP+ and benzyl alcohol and xylitol was monitored by an increase in NADPH fluorescence (ex: 340 nm; em: 460 nm) using a... | Bioorg Chem 34: 424-44 (2006) Article DOI: 10.1016/j.bioorg.2006.09.004 BindingDB Entry DOI: 10.7270/Q2SN076W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50010437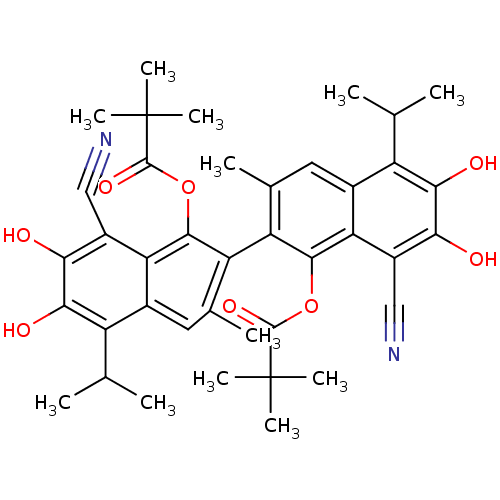 (2,2-Dimethyl-propionic acid 8,8'-dicyano-1'-(2,2-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against Aldose reductase from human placenta | J Med Chem 34: 3301-5 (1991) BindingDB Entry DOI: 10.7270/Q2VM4B7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50010450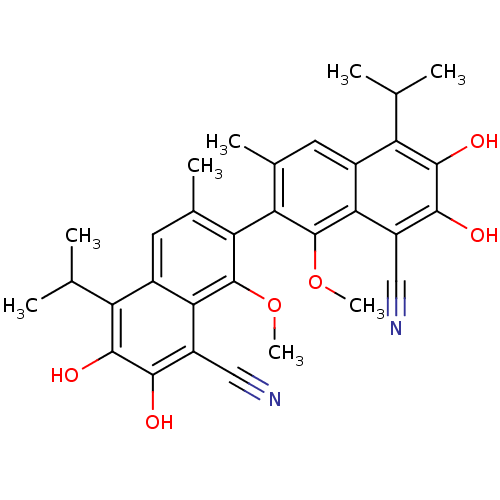 (6,7,6',7'-Tetrahydroxy-5,5'-diisopropyl-1,1'-dimet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against Aldose reductase from human placenta | J Med Chem 34: 3301-5 (1991) BindingDB Entry DOI: 10.7270/Q2VM4B7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50010440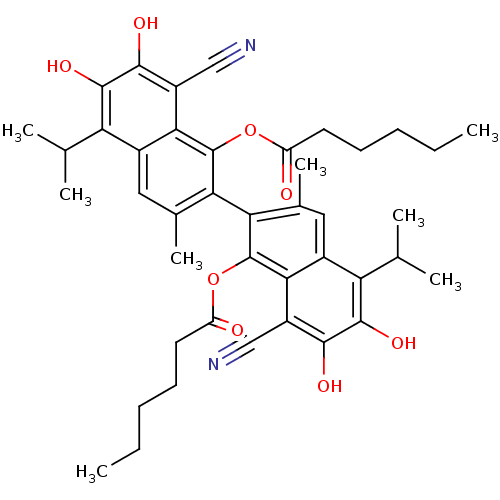 (CHEMBL326383 | Hexanoic acid 8,8'-dicyano-1'-hexan...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against aldose reductase from human placenta | J Med Chem 34: 3301-5 (1991) BindingDB Entry DOI: 10.7270/Q2VM4B7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16426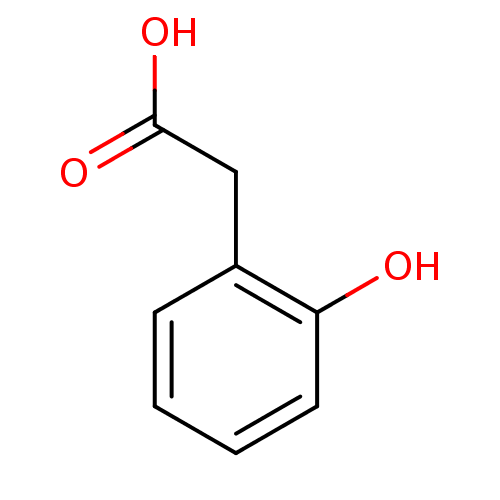 (2-(2-hydroxyphenyl)acetic acid | 2-Hydroxyphenylac...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 3.50E+3 | -31.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Medical College of Wiscosin | Assay Description The production of NADPH from NADP+ and benzyl alcohol and xylitol was monitored by an increase in NADPH fluorescence (ex: 340 nm; em: 460 nm) using a... | Bioorg Chem 34: 424-44 (2006) Article DOI: 10.1016/j.bioorg.2006.09.004 BindingDB Entry DOI: 10.7270/Q2SN076W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16441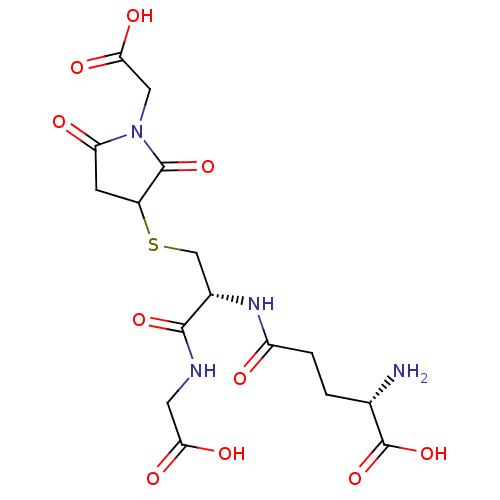 ((2S)-2-amino-4-{[(1R)-2-{[1-(carboxymethyl)-2,5-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wiscosin | Assay Description The production of NADPH from NADP+ and benzyl alcohol and xylitol was monitored by an increase in NADPH fluorescence (ex: 340 nm; em: 460 nm) using a... | Bioorg Chem 34: 424-44 (2006) Article DOI: 10.1016/j.bioorg.2006.09.004 BindingDB Entry DOI: 10.7270/Q2SN076W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50010447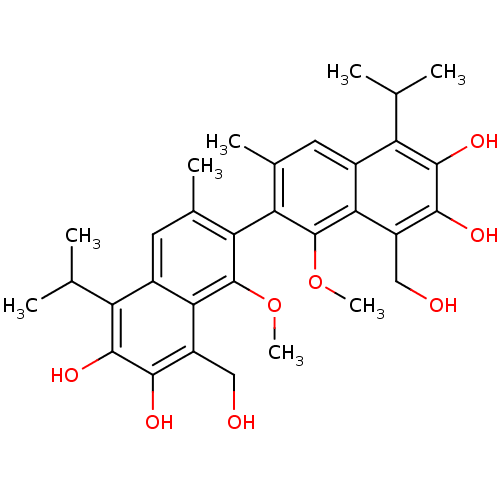 (8,8'-Bis-hydroxymethyl-5,5'-diisopropyl-1,1'-dimet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against Aldose reductase from human placenta | J Med Chem 34: 3301-5 (1991) BindingDB Entry DOI: 10.7270/Q2VM4B7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16429 (2,6-Dichlorophenylacetic acid | 2-(2,6-dichlorophe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 4.40E+3 | -30.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Medical College of Wiscosin | Assay Description The production of NADPH from NADP+ and benzyl alcohol and xylitol was monitored by an increase in NADPH fluorescence (ex: 340 nm; em: 460 nm) using a... | Bioorg Chem 34: 424-44 (2006) Article DOI: 10.1016/j.bioorg.2006.09.004 BindingDB Entry DOI: 10.7270/Q2SN076W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50010444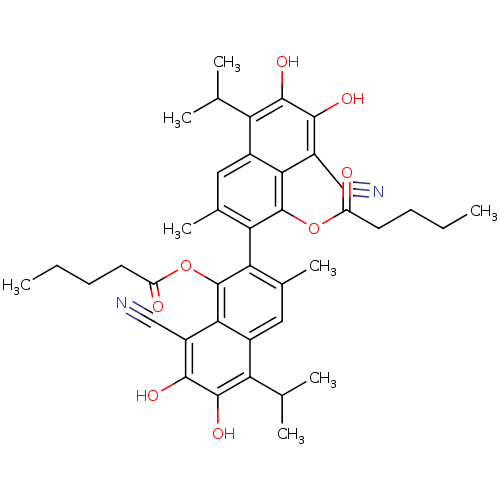 (CHEMBL52639 | Pentanoic acid 8,8'-dicyano-6,7,6',7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against aldose reductase from human placenta | J Med Chem 34: 3301-5 (1991) BindingDB Entry DOI: 10.7270/Q2VM4B7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM50010334 (CHEMBL3251378) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of beef brain NADP-dependent aldehyde reductase using p-nitrobenzaldehyde by competitive inhibition assay | J Med Chem 22: 1011-4 (1979) BindingDB Entry DOI: 10.7270/Q21C1ZD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50010435 (Butyric acid 1'-butyryloxy-8,8'-dicyano-6,7,6',7'-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against aldose reductase from human placenta | J Med Chem 34: 3301-5 (1991) BindingDB Entry DOI: 10.7270/Q2VM4B7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Non-competitive inhibition of recombinant human N-terminal His6-tagged AKR1B10 expressed in Escherichia coli BL21 cells using all-trans-retinal as su... | J Nat Prod 78: 2666-74 (2015) Article DOI: 10.1021/acs.jnatprod.5b00616 BindingDB Entry DOI: 10.7270/Q2XG9V5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16431 (2-(naphthalen-2-yl)acetic acid | 2-Napthylacetic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.20E+3 | -29.7 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Medical College of Wiscosin | Assay Description The production of NADPH from NADP+ and benzyl alcohol and xylitol was monitored by an increase in NADPH fluorescence (ex: 340 nm; em: 460 nm) using a... | Bioorg Chem 34: 424-44 (2006) Article DOI: 10.1016/j.bioorg.2006.09.004 BindingDB Entry DOI: 10.7270/Q2SN076W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16440 (2-{3-[(2-amino-3-methoxy-3-oxopropyl)sulfanyl]-2,5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wiscosin | Assay Description The production of NADPH from NADP+ and benzyl alcohol and xylitol was monitored by an increase in NADPH fluorescence (ex: 340 nm; em: 460 nm) using a... | Bioorg Chem 34: 424-44 (2006) Article DOI: 10.1016/j.bioorg.2006.09.004 BindingDB Entry DOI: 10.7270/Q2SN076W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50010439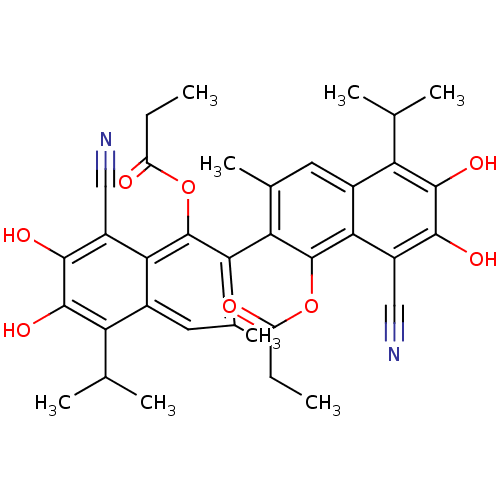 (CHEMBL297483 | Propionic acid 8,8'-dicyano-6,7,6',...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against aldose reductase from human placenta | J Med Chem 34: 3301-5 (1991) BindingDB Entry DOI: 10.7270/Q2VM4B7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3258 total ) | Next | Last >> |