

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(3) dopamine receptor (Homo sapiens) | BDBM50601552 (CHEMBL5183205) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 0.0258 | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00692 BindingDB Entry DOI: 10.7270/Q2MP57CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM50601583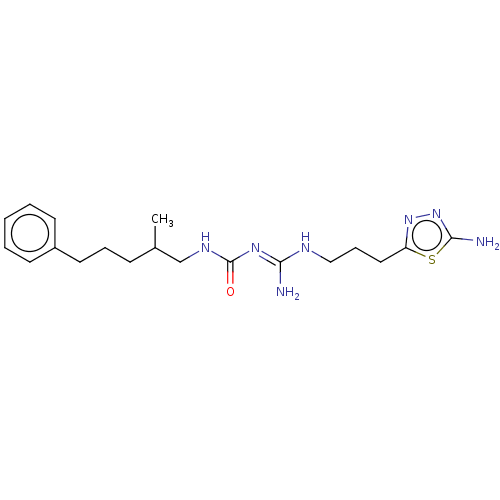 (CHEMBL5198795) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 0.0258 | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00692 BindingDB Entry DOI: 10.7270/Q2MP57CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM50601579 (CHEMBL5202592) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 0.0258 | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00692 BindingDB Entry DOI: 10.7270/Q2MP57CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM50601577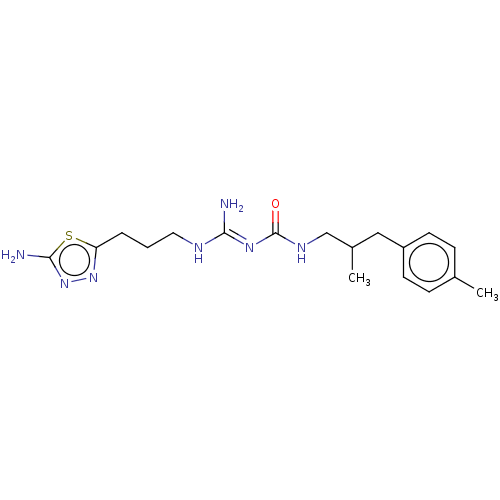 (CHEMBL5180504) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 0.0258 | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00692 BindingDB Entry DOI: 10.7270/Q2MP57CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM50601576 (CHEMBL5184911) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 0.0258 | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00692 BindingDB Entry DOI: 10.7270/Q2MP57CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM50601574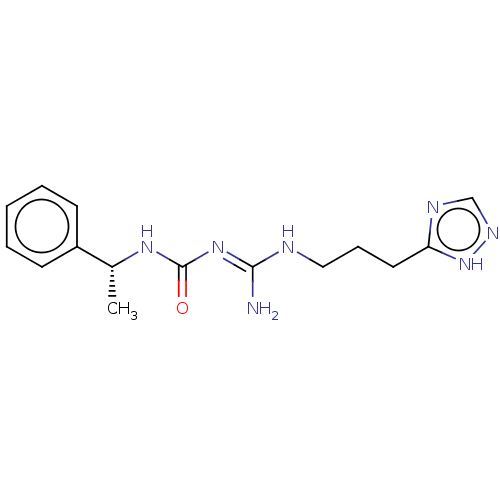 (CHEMBL5201074) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 0.0258 | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00692 BindingDB Entry DOI: 10.7270/Q2MP57CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM50601573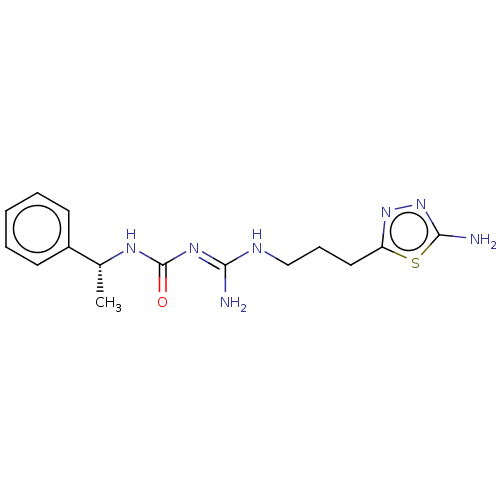 (CHEMBL5208845) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 0.0258 | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00692 BindingDB Entry DOI: 10.7270/Q2MP57CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM50601568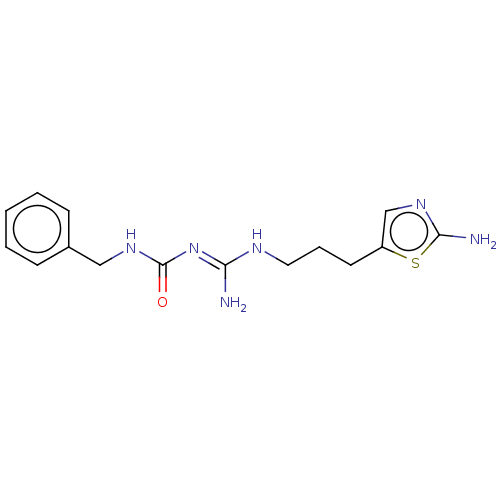 (CHEMBL5178472) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 0.0258 | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00692 BindingDB Entry DOI: 10.7270/Q2MP57CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM50601567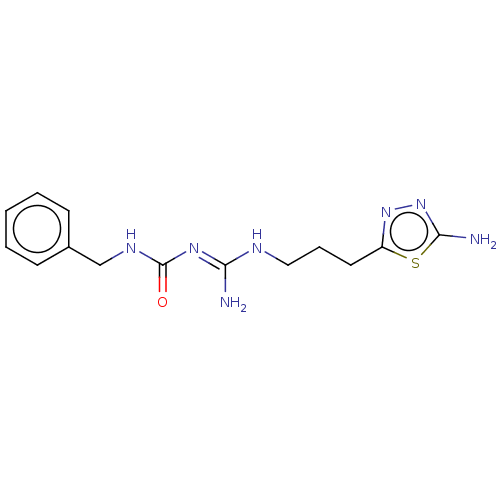 (CHEMBL5206565) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 0.0258 | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00692 BindingDB Entry DOI: 10.7270/Q2MP57CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM50116766 ((-)-Pramipexole | (6S)-N(6)-propyl-4,5,6,7-tetrahy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 0.0258 | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00692 BindingDB Entry DOI: 10.7270/Q2MP57CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM50601551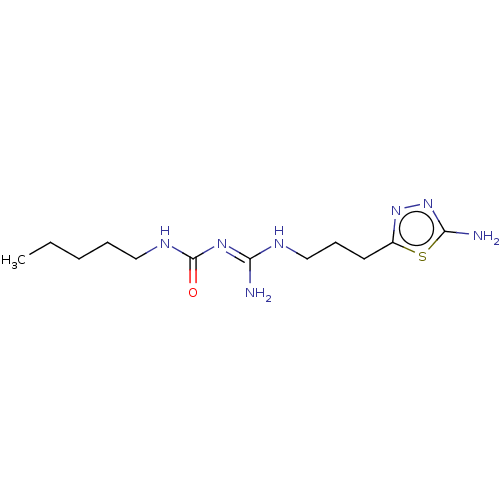 (CHEMBL5207281) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 0.0258 | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00692 BindingDB Entry DOI: 10.7270/Q2MP57CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM50601589 (CHEMBL5176229) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 0.0258 | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00692 BindingDB Entry DOI: 10.7270/Q2MP57CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM50601556 (CHEMBL5200771) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 0.0258 | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00692 BindingDB Entry DOI: 10.7270/Q2MP57CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM50601563 (CHEMBL5204599) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 0.0258 | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00692 BindingDB Entry DOI: 10.7270/Q2MP57CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50343785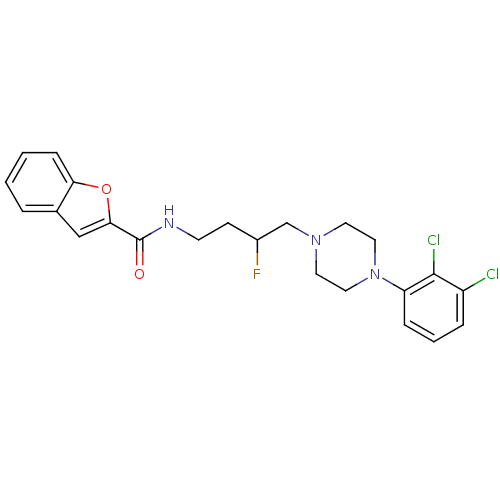 (CHEMBL1774386 | N-(4-(4-(2,3-Dichlorophenyl)pipera...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse-Intramural Research Program Curated by ChEMBL | Assay Description Binding affinity to wild type human dopamine D3 receptor expressed in HEK293 cells | J Med Chem 54: 3581-94 (2011) Article DOI: 10.1021/jm200288r BindingDB Entry DOI: 10.7270/Q25M661K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50043603 (7-[(3-Iodo-allyl)-propyl-amino]-5,6,7,8-tetrahydro...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity was tested against dopamine receptor D3 in Sf9 cells | J Med Chem 36: 4308-12 (1994) BindingDB Entry DOI: 10.7270/Q2TB15ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50487259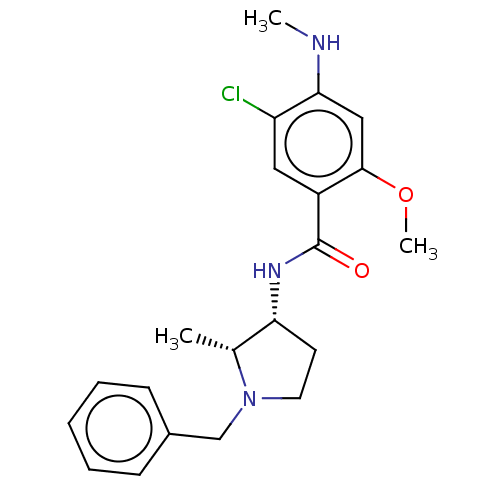 (CHEBI:64219 | [3H]NEMONAPRIDE) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article | n/a | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to Rattus norvegicus (rat) chimeric dopamine D3 trunk/D3 tail receptor transfected in african green monkey COS7 cells after 1 hr by ... | Citation and Details Article DOI: 10.1007/s00044-004-0006-x BindingDB Entry DOI: 10.7270/Q2377CM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50487259 (CHEBI:64219 | [3H]NEMONAPRIDE) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article | n/a | n/a | n/a | 0.0640 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to Rattus norvegicus (rat) wild type dopamine D3 receptor transfected in african green monkey COS7 cells after 1 hr by beta scintill... | Citation and Details Article DOI: 10.1007/s00044-004-0006-x BindingDB Entry DOI: 10.7270/Q2377CM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50038276 ((R,S) 7-[(3-Iodo-allyl)-propyl-amino]-5,6,7,8-tetr...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity was tested against dopamine receptor D3 in the absence of NaCl | J Med Chem 36: 4308-12 (1994) BindingDB Entry DOI: 10.7270/Q2TB15ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50038276 ((R,S) 7-[(3-Iodo-allyl)-propyl-amino]-5,6,7,8-tetr...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Binding affinity was tested against dopamine receptor D3 in rat striatal membarane homogenate | J Med Chem 36: 4308-12 (1994) BindingDB Entry DOI: 10.7270/Q2TB15ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50020222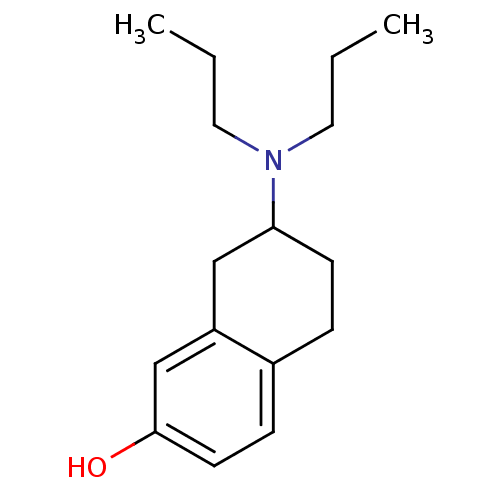 ((+/-)-7-(dipropylamino)-5,6,7,8-tetrahydronaphthal...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania Curated by ChEMBL | Assay Description Tested for binding affinity against dopamine receptor D3 | J Med Chem 36: 4308-12 (1994) BindingDB Entry DOI: 10.7270/Q2TB15ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50336701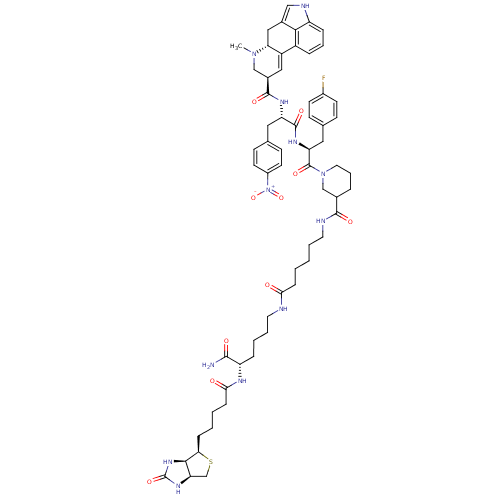 ((6aR,9R)-N-((2S)-1-((2S)-1-(3-(6-((S)-6-amino-6-ox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human dopamine D3 receptor high binding affinity site expressed in CHO cells | J Med Chem 54: 1080-90 (2011) Article DOI: 10.1021/jm101566d BindingDB Entry DOI: 10.7270/Q2QF8T5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50336701 ((6aR,9R)-N-((2S)-1-((2S)-1-(3-(6-((S)-6-amino-6-ox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Displacement of [3H]-raclopride from human dopamine D3 receptor low binding affinity site expressed in CHO cells | J Med Chem 54: 1080-90 (2011) Article DOI: 10.1021/jm101566d BindingDB Entry DOI: 10.7270/Q2QF8T5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476758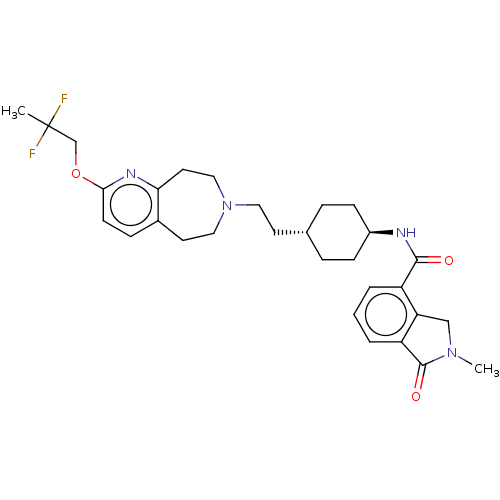 (US10870660, Compound III-024 | US11345716, Compoun...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476918 (US10870660, Compound II-057) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593894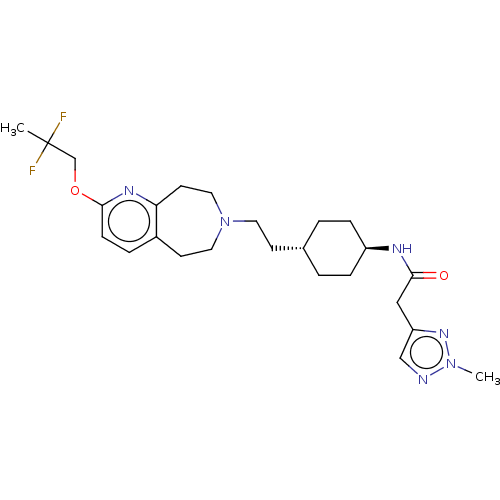 (US11578084, Compound I'-42) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50063292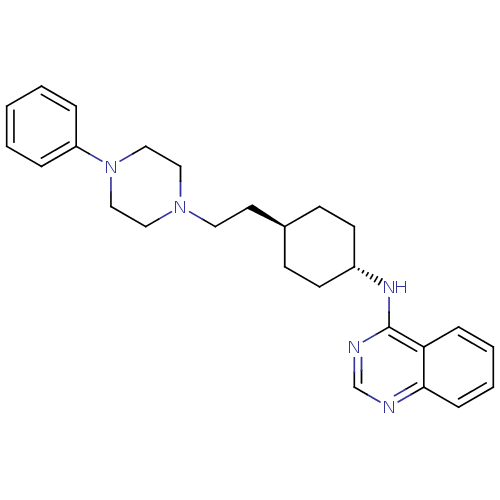 (CHEMBL349426 | {4-[2-(4-Phenyl-piperazin-1-yl)-eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity determined by measuring displacement of [3H]-spiperone from cloned Human Dopamine receptor D3 in CHO-K1 cells | J Med Chem 41: 760-71 (1998) Article DOI: 10.1021/jm9707378 BindingDB Entry DOI: 10.7270/Q20G3J97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50290221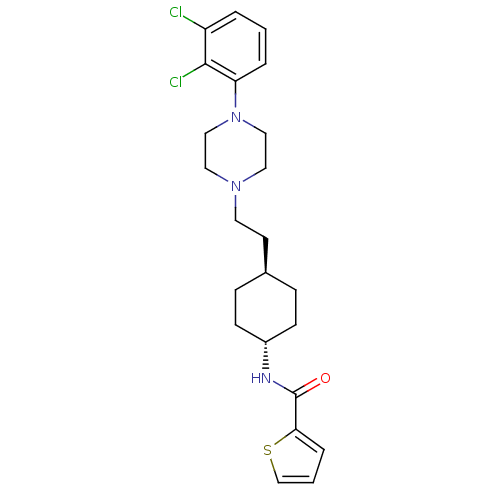 (CHEMBL80919 | Thiophene-2-carboxylic acid (4-{2-[4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]N-0437 from human dopamine D2 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476915 (US10870660, Compound II-047) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593791 (US11578084, Compound I-074) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476764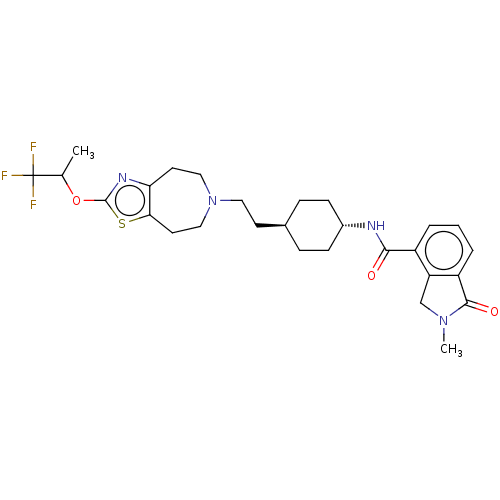 (US10870660, Compound III-064 | US11345716, Compoun...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50341508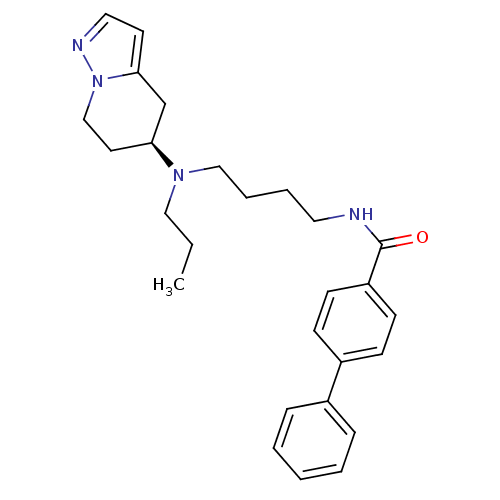 ((S)-N-(4-(4-Phenylbenzoylamino)butyl)-N-propyl-5-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Displacement of [3H]7-OH-DPAT from human dopamine D3 receptor expressed in CHO cells after 60 mins | J Med Chem 54: 2477-91 (2011) Article DOI: 10.1021/jm101639t BindingDB Entry DOI: 10.7270/Q2V1253W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50207116 (CHEMBL3905247 | US9550741, I-4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593929 (US11578084, Compound I-157) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593940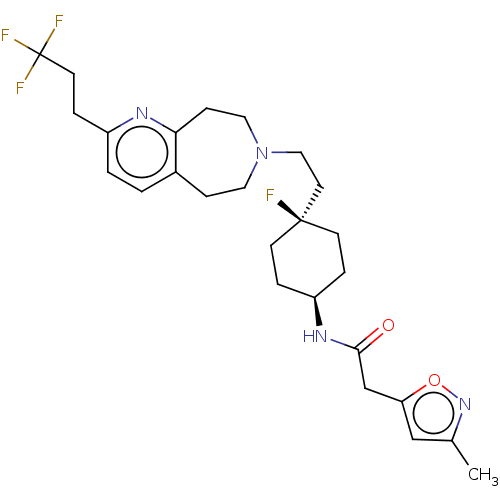 (US11578084, Compound III-3) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593840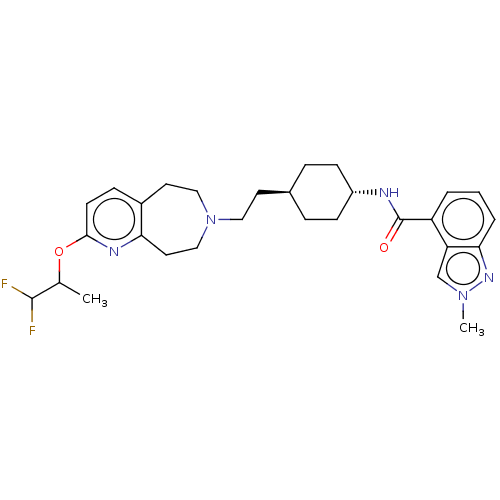 (US11578084, Compound I-123 | US11578084, Compound ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476836 (US10870660, Compound III-581 | US11345716, Compoun...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593735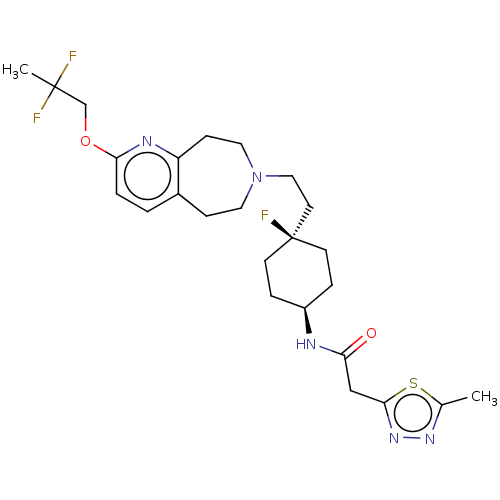 (US11578084, Compound I-018) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593733 (US11578084, Compound I-016) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50099807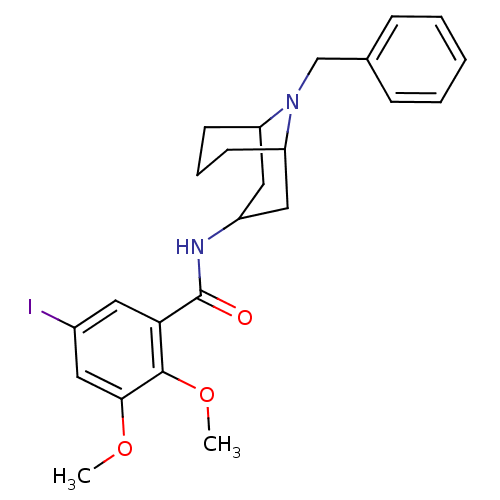 (CHEMBL54866 | N-(9-Benzyl-9-aza-bicyclo[3.3.1]non-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wake Forest University School of Medicine Curated by ChEMBL | Assay Description Binding affinity for dopamine receptor D3 expressed in Sf9 cells using [125I]-IABN the radioligand. | J Med Chem 44: 1815-26 (2001) BindingDB Entry DOI: 10.7270/Q2NK3DBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50099821 (4-Bromo-1-methoxy-naphthalene-2-carboxylic acid (9...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wake Forest University School of Medicine Curated by ChEMBL | Assay Description Binding affinity for dopamine receptor D3 expressed in Sf9 cells using [125I]-IABN the radioligand. | J Med Chem 44: 1815-26 (2001) BindingDB Entry DOI: 10.7270/Q2NK3DBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50008735 ((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adamed Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]methylspiperone from human recombinant D3 receptor expressed in CHO cells | J Med Chem 57: 4543-57 (2014) Article DOI: 10.1021/jm401895u BindingDB Entry DOI: 10.7270/Q2N29ZHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50008735 ((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Displacement of [3H]methylspiperone from human dopamine D3 receptor expressed in CHO cells | Eur J Med Chem 92: 221-35 (2015) Article DOI: 10.1016/j.ejmech.2014.12.045 BindingDB Entry DOI: 10.7270/Q2Q241XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50207143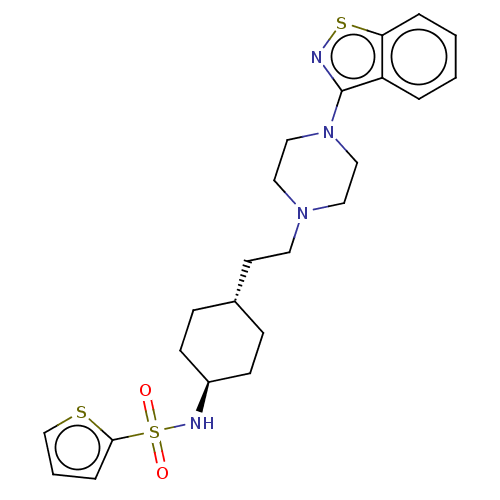 (CHEMBL3966842 | US9550741, II-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593890 (US11578084, Compound I'-38) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50253328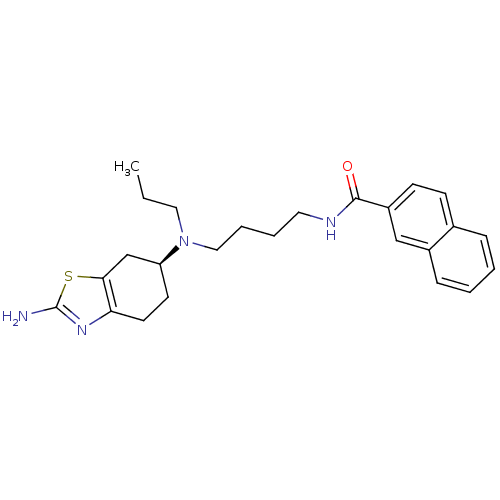 ((S)-N-(4-((2-amino-4,5,6,7-tetrahydrobenzo[d]thiaz...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Displacement of [3H]PD128907 from dopamine D3 receptor in Sprague-Dawley rat ventral striatum | J Med Chem 51: 5905-8 (2008) Article DOI: 10.1021/jm800471h BindingDB Entry DOI: 10.7270/Q2FN161H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50207162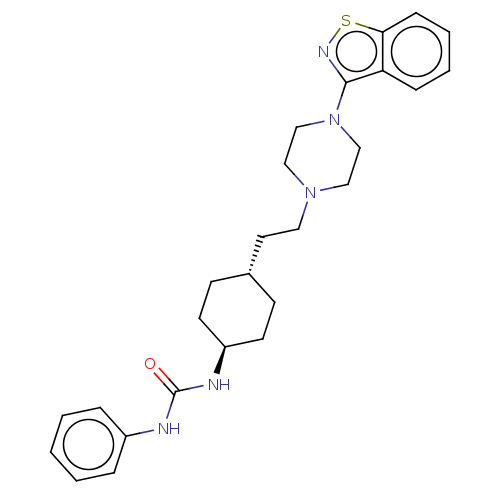 (CHEMBL3918755 | US9550741, IV-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593802 (US11578084, Compound I-085) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50207155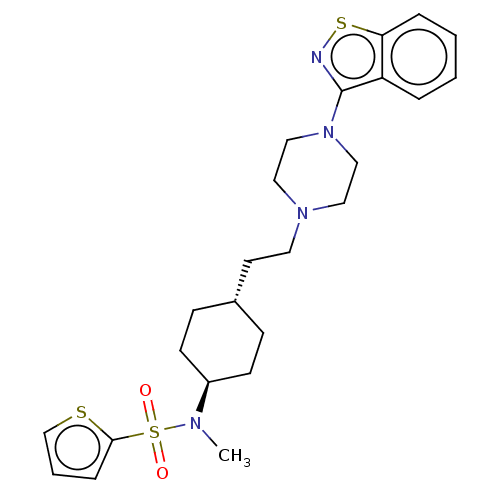 (CHEMBL3895540) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50207141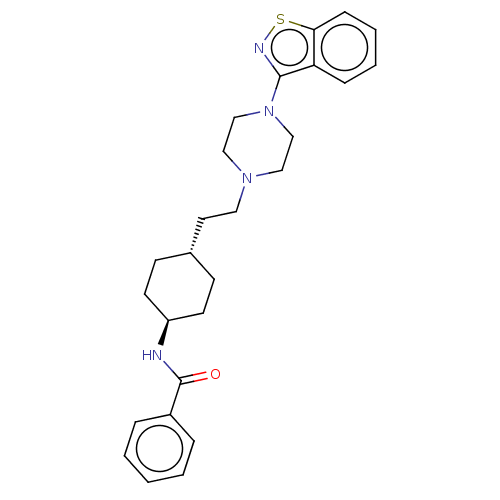 (CHEMBL3920252) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 9656 total ) | Next | Last >> |