

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM92485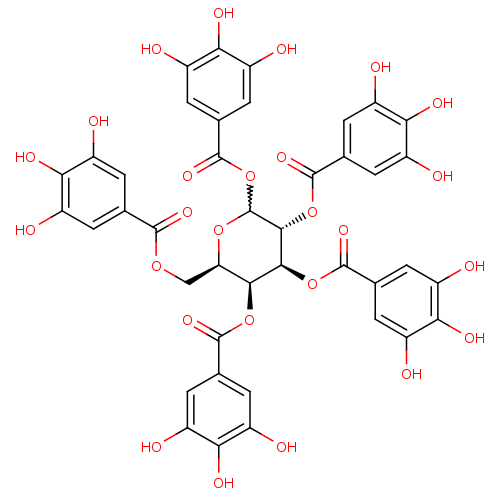 (CDE-066 | US9120744, CDE-066) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 3.10 | n/a | n/a | n/a | 5.0 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To establish binding constants for the compounds/drugs to PAI-1, an indirect approach using surface plasmon resonance (SPR) was employed. Varying con... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM92485 (CDE-066 | US9120744, CDE-066) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.10 | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan | Assay Description Enzyme activity assay using human and murine PAI-1. | J Biol Chem 285: 7892-902 (2010) Article DOI: 10.1074/jbc.M109.067967 BindingDB Entry DOI: 10.7270/Q2ZC81FC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM92486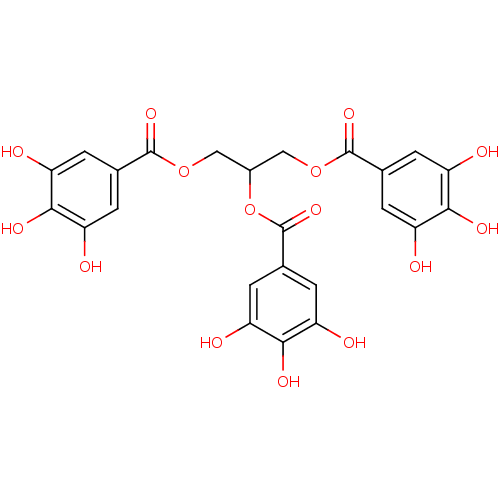 (CDE-082 | US9120744, CDE-082) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 5.30 | n/a | n/a | n/a | 5.0 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To establish binding constants for the compounds/drugs to PAI-1, an indirect approach using surface plasmon resonance (SPR) was employed. Varying con... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM92486 (CDE-082 | US9120744, CDE-082) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 5.30 | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan | Assay Description Enzyme activity assay using human and murine PAI-1. | J Biol Chem 285: 7892-902 (2010) Article DOI: 10.1074/jbc.M109.067967 BindingDB Entry DOI: 10.7270/Q2ZC81FC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM92482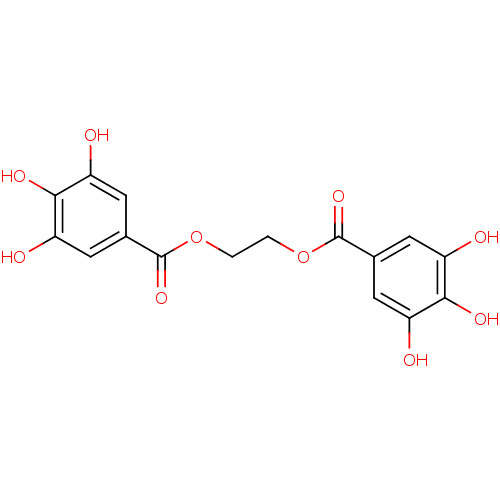 (CDE-008 | US9120744, CDE-008) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 23 | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan | Assay Description Enzyme activity assay using human and murine PAI-1. | J Biol Chem 285: 7892-902 (2010) Article DOI: 10.1074/jbc.M109.067967 BindingDB Entry DOI: 10.7270/Q2ZC81FC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM92482 (CDE-008 | US9120744, CDE-008) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 23 | n/a | n/a | n/a | 5.0 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To establish binding constants for the compounds/drugs to PAI-1, an indirect approach using surface plasmon resonance (SPR) was employed. Varying con... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM92487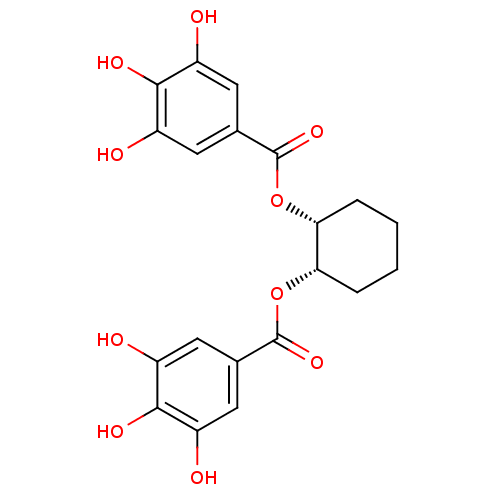 (CDE-031 | US9120744, CDE-031) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 31 | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan | Assay Description Enzyme activity assay using human and murine PAI-1. | J Biol Chem 285: 7892-902 (2010) Article DOI: 10.1074/jbc.M109.067967 BindingDB Entry DOI: 10.7270/Q2ZC81FC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM92487 (CDE-031 | US9120744, CDE-031) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 31 | n/a | n/a | n/a | 5.0 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To establish binding constants for the compounds/drugs to PAI-1, an indirect approach using surface plasmon resonance (SPR) was employed. Varying con... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM92484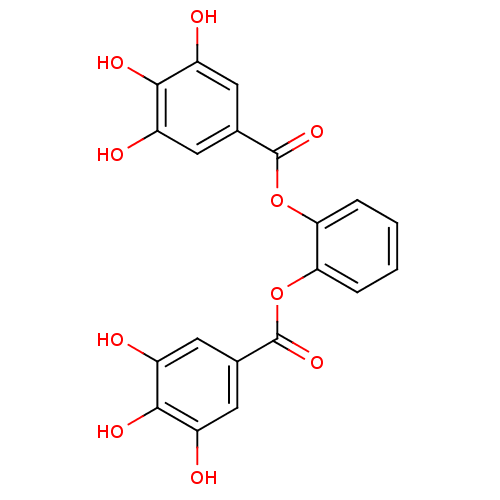 (CDE-056 | US9120744, CDE-056) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 51 | n/a | n/a | n/a | 5.0 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To establish binding constants for the compounds/drugs to PAI-1, an indirect approach using surface plasmon resonance (SPR) was employed. Varying con... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM92484 (CDE-056 | US9120744, CDE-056) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 51 | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan | Assay Description Enzyme activity assay using human and murine PAI-1. | J Biol Chem 285: 7892-902 (2010) Article DOI: 10.1074/jbc.M109.067967 BindingDB Entry DOI: 10.7270/Q2ZC81FC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM92483 (CDE-034 | US9120744, CDE-034) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 67 | n/a | n/a | n/a | 5.0 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To establish binding constants for the compounds/drugs to PAI-1, an indirect approach using surface plasmon resonance (SPR) was employed. Varying con... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM92483 (CDE-034 | US9120744, CDE-034) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 67 | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan | Assay Description Enzyme activity assay using human and murine PAI-1. | J Biol Chem 285: 7892-902 (2010) Article DOI: 10.1074/jbc.M109.067967 BindingDB Entry DOI: 10.7270/Q2ZC81FC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Rattus norvegicus) | BDBM50149275 (2-(1-benzyl-5-(4-(trifluoromethoxy)phenyl)-1H-indo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity of the compound to towards NBD-labeled S119C Plasminogen activator inhibitor-1 mutant in rat | J Med Chem 47: 3491-4 (2004) Article DOI: 10.1021/jm049766q BindingDB Entry DOI: 10.7270/Q2D21X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Rattus norvegicus) | BDBM50149275 (2-(1-benzyl-5-(4-(trifluoromethoxy)phenyl)-1H-indo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity of compound towards latent Plasminogen activator inhibitor-1 expressed as apparent Kd | J Med Chem 47: 3491-4 (2004) Article DOI: 10.1021/jm049766q BindingDB Entry DOI: 10.7270/Q2D21X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50149275 (2-(1-benzyl-5-(4-(trifluoromethoxy)phenyl)-1H-indo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical School Curated by ChEMBL | Assay Description Binding affinity to human immobilized PAI1 by surface plasmon resonance | J Biol Chem 282: 9288-96 (2007) Article DOI: 10.1074/jbc.M611642200 BindingDB Entry DOI: 10.7270/Q2FF3S51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM92479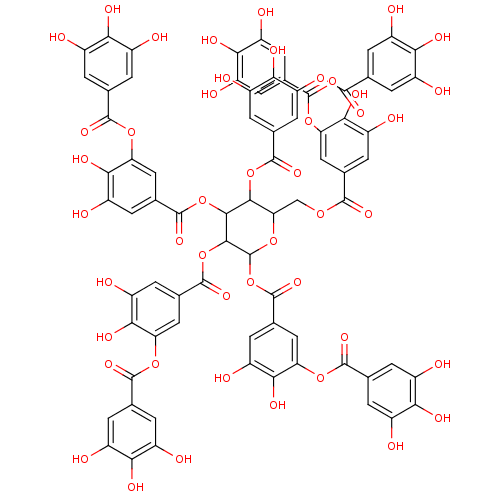 (Tannic Acid, A | Tannic acid) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan | Assay Description Enzyme activity assay using human and murine PAI-1. | J Biol Chem 285: 7892-902 (2010) Article DOI: 10.1074/jbc.M109.067967 BindingDB Entry DOI: 10.7270/Q2ZC81FC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50070942 ((-)-Epigallocatechin gallate | (-)-Epigallocatechi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan | Assay Description Enzyme activity assay using human and murine PAI-1. | J Biol Chem 285: 7892-902 (2010) Article DOI: 10.1074/jbc.M109.067967 BindingDB Entry DOI: 10.7270/Q2ZC81FC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM92480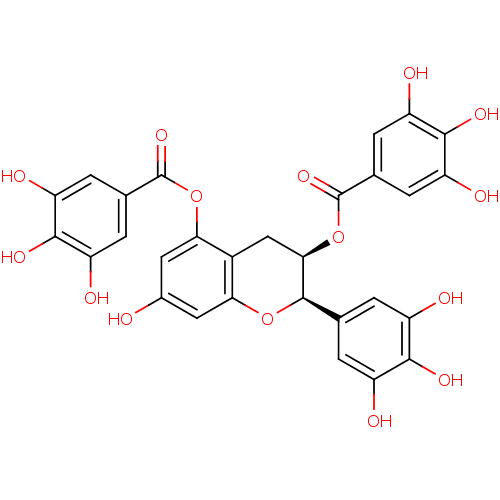 (Epigallocatechin-3,5-Digallate, C) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan | Assay Description Enzyme activity assay using human and murine PAI-1. | J Biol Chem 285: 7892-902 (2010) Article DOI: 10.1074/jbc.M109.067967 BindingDB Entry DOI: 10.7270/Q2ZC81FC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM31712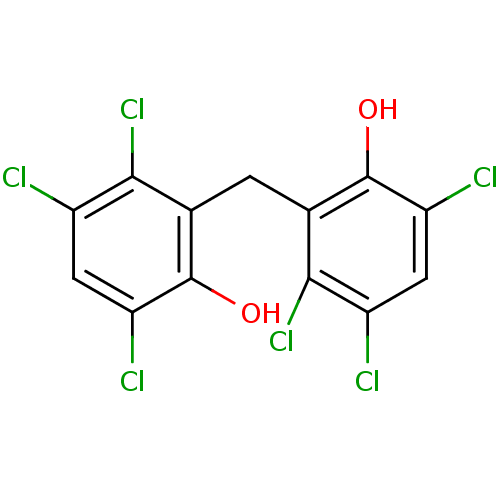 (HEXACHLOROPHENE | Hexach-lorophene | MLS000028433 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan | Assay Description Enzyme activity assay using human and murine PAI-1. | J Biol Chem 285: 7892-902 (2010) Article DOI: 10.1074/jbc.M109.067967 BindingDB Entry DOI: 10.7270/Q2ZC81FC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50041419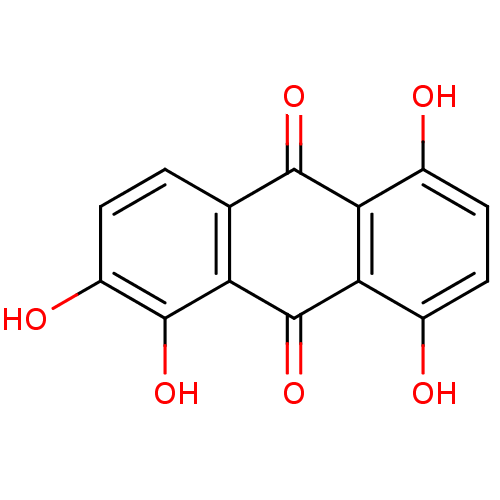 (1,2,5,8-tetrahydroxy-9,10-anthracenedione | 1,2,5,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan | Assay Description Enzyme activity assay using human and murine PAI-1. | J Biol Chem 285: 7892-902 (2010) Article DOI: 10.1074/jbc.M109.067967 BindingDB Entry DOI: 10.7270/Q2ZC81FC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50085536 (3,4,5-Trihydroxybenzoate, X | 3,4,5-trihydroxybenz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan | Assay Description Enzyme activity assay using human and murine PAI-1. | J Biol Chem 285: 7892-902 (2010) Article DOI: 10.1074/jbc.M109.067967 BindingDB Entry DOI: 10.7270/Q2ZC81FC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM92481 (Sennoside A, G) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10E+4 | n/a | n/a | n/a | n/a | 7.8 | 23 |
University of Michigan | Assay Description Enzyme activity assay using human and murine PAI-1. | J Biol Chem 285: 7892-902 (2010) Article DOI: 10.1074/jbc.M109.067967 BindingDB Entry DOI: 10.7270/Q2ZC81FC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM175527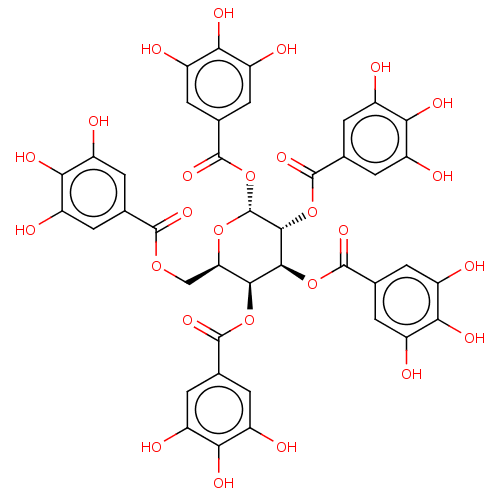 (US9120744, CDE-003) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM175526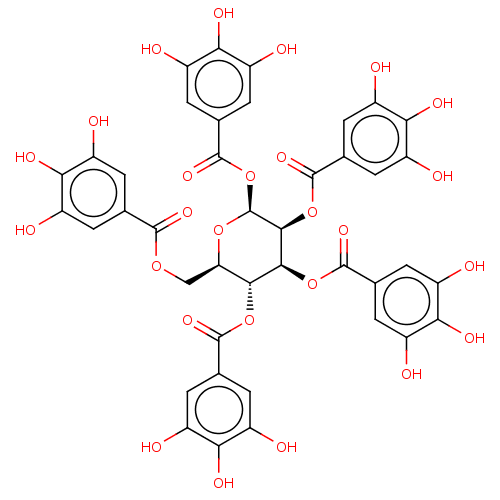 (US9120744, CDE-002) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM175528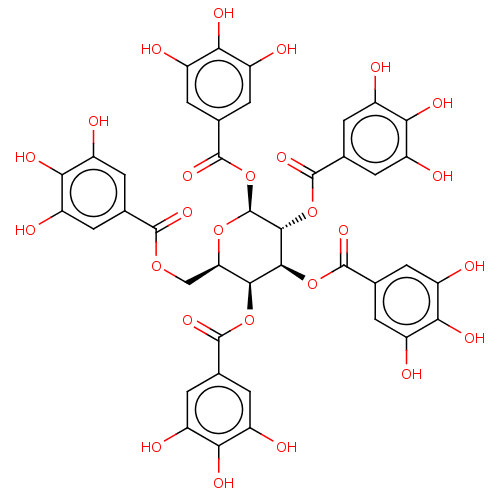 (US9120744, CDE-004) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM175525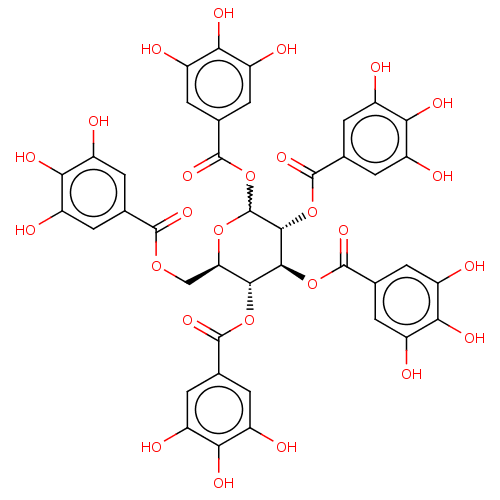 (US9120744, CDE-001 (or 073)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM92485 (CDE-066 | US9120744, CDE-066) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM175546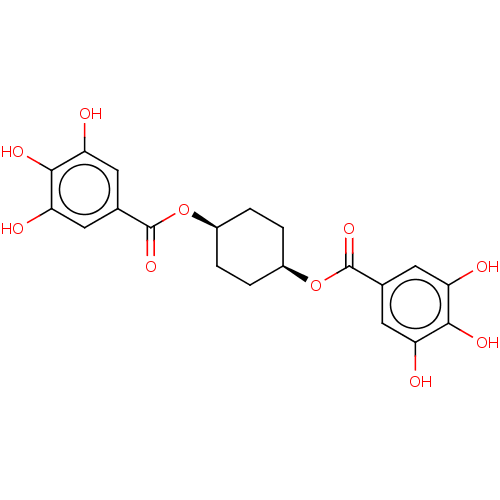 (US9120744, CDE-061) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM175544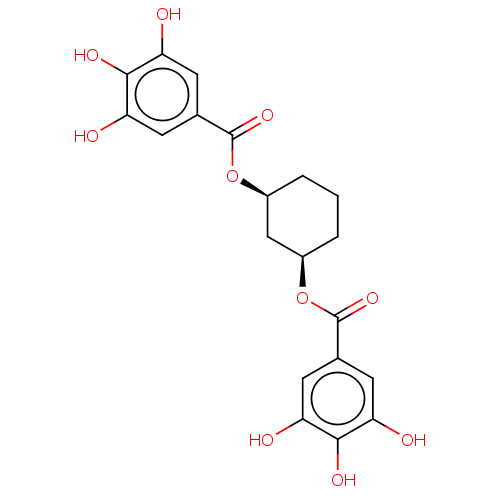 (US9120744, CDE-058) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM175558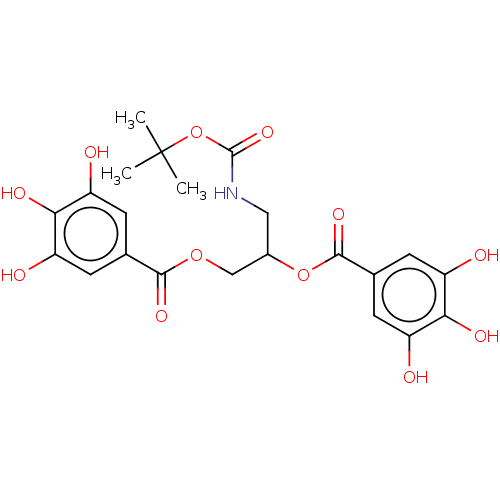 (US9120744, CDE-075) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 70 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM175548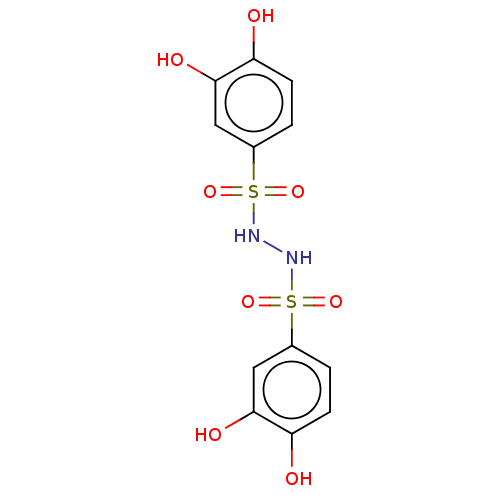 (US9120744, CDE-059) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 90 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM92487 (CDE-031 | US9120744, CDE-031) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 280 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM92482 (CDE-008 | US9120744, CDE-008) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 370 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM92483 (CDE-034 | US9120744, CDE-034) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM175552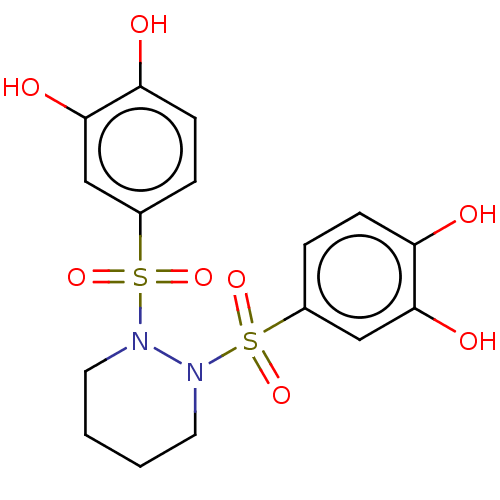 (US9120744, CDE-074) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.71E+3 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM92484 (CDE-056 | US9120744, CDE-056) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.96E+3 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM175534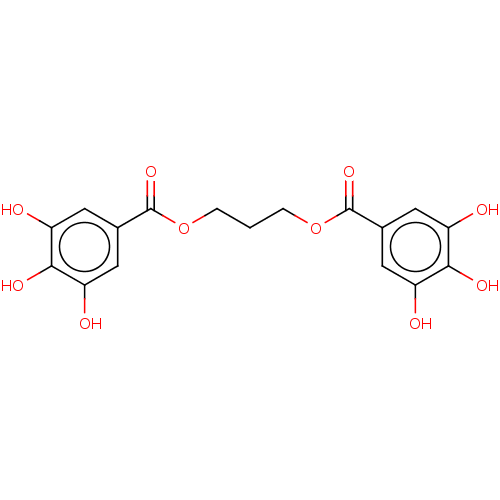 (US9120744, CDE-013) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7.85E+3 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM50310301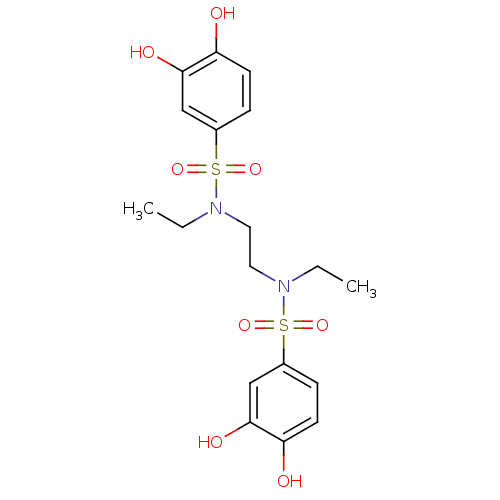 (CHEMBL599742 | N,N'-(ethane-1,2-diyl)bis(N-ethyl-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM175542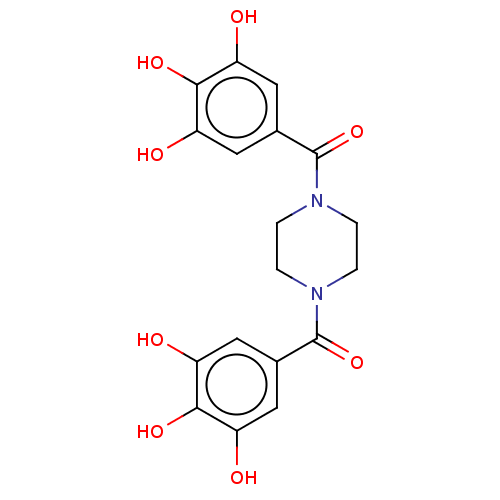 (US9120744, CDE-055) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.75E+4 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM175537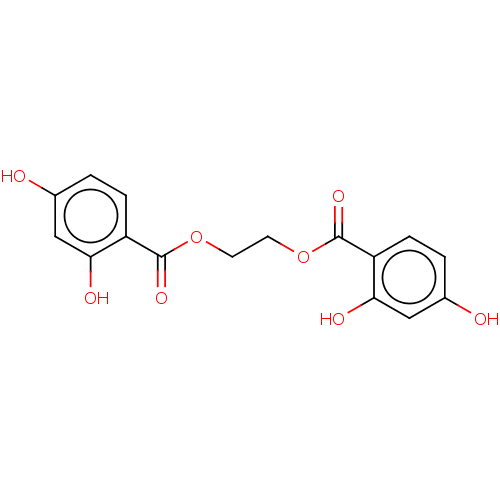 (US9120744, CDE-051) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.87E+4 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM175549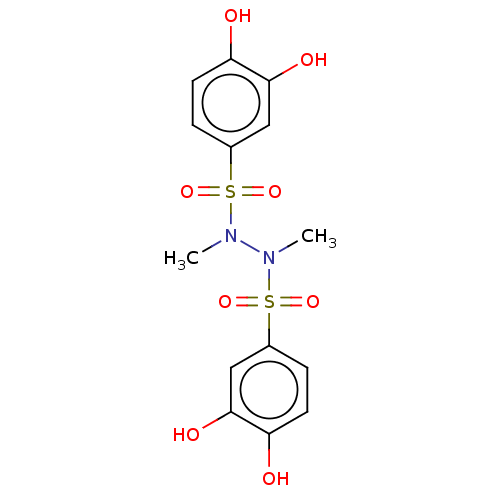 (US9120744, CDE-068) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.27E+4 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM175553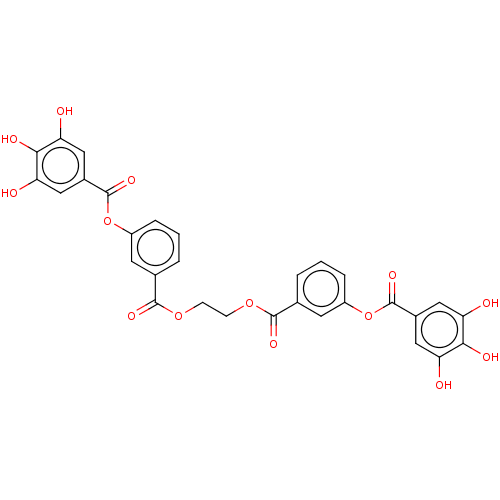 (US9120744, CDE-041) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.79E+4 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM175557 (US9120744, CDE-067) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.83E+4 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM175531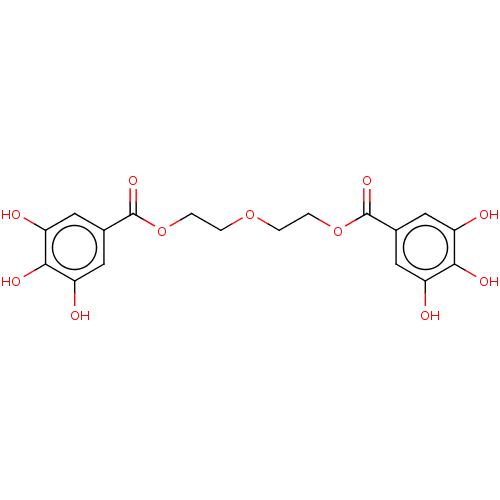 (US9120744, CDE-009) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.93E+4 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM175551 (US9120744, CDE-072) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.04E+5 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM175554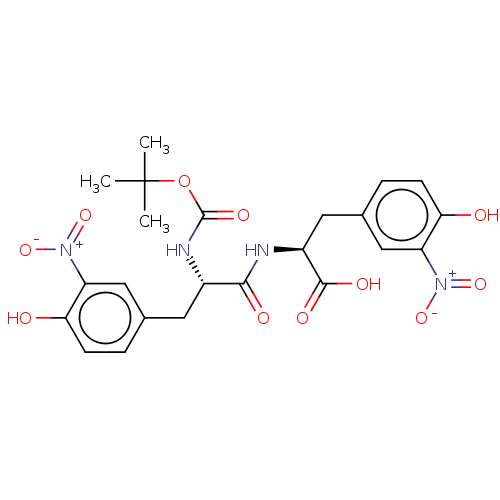 (US9120744, CDE-035) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.46E+5 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM175545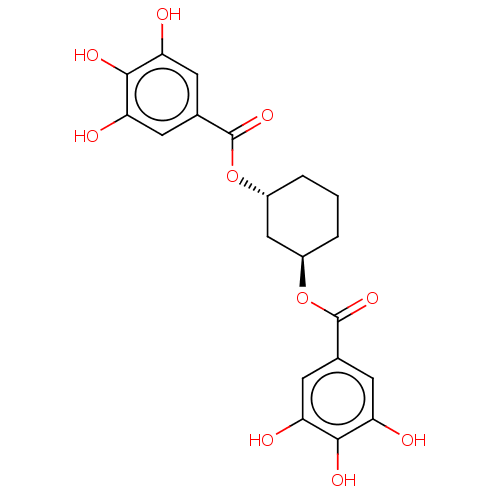 (US9120744, CDE-057) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.95E+5 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM175532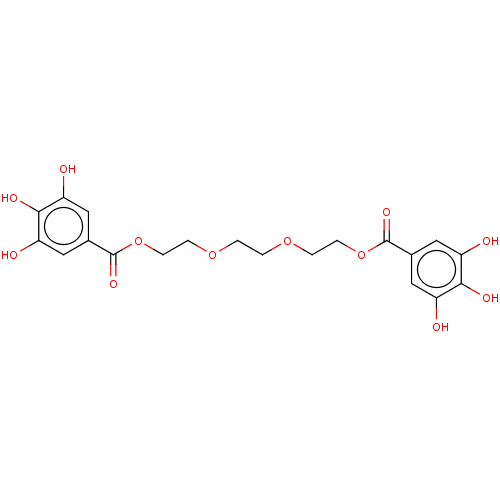 (US9120744, CDE-012) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.96E+5 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM175538 (US9120744, CDE-060) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.36E+5 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen activator inhibitor 1 (Homo sapiens (Human)) | BDBM175561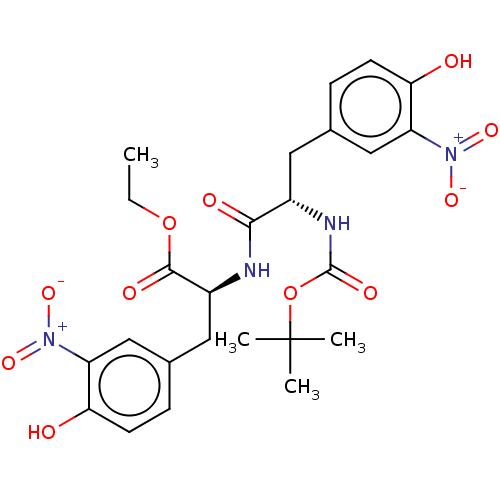 (US9120744, CDE-033) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.46E+5 | n/a | n/a | n/a | n/a | 7.8 | 23 |
THE REGENTS OF THE UNIVERSITY OF MICHIGAN; EASTERN MICHIGAN UNIVERSITY US Patent | Assay Description To determine the efficacy of various synthesized PAI-1 inhibitor compounds, a fluorometric plate assay was carried out to measure the half maximal in... | US Patent US9120744 (2015) BindingDB Entry DOI: 10.7270/Q2QF8RN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 375 total ) | Next | Last >> |