

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thiopurine S-methyltransferase (Homo sapiens (Human)) | BDBM50378739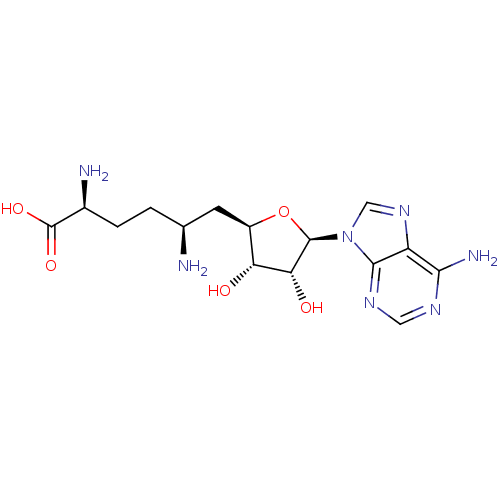 (SINEFUNGIN | jm2c00120, Sinefungin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutical Research and Development, LLC Curated by ChEMBL | Assay Description Binding affinity to thiopurine methyltransferase (unknown origin) by NMR analysis | J Med Chem 57: 7819-37 (2014) Article DOI: 10.1021/jm500325k BindingDB Entry DOI: 10.7270/Q2JW8GH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiopurine S-methyltransferase (Homo sapiens (Human)) | BDBM50530712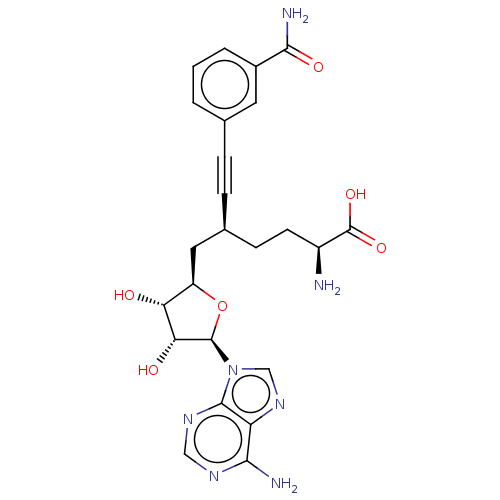 (CHEMBL4591248) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd. Curated by ChEMBL | Assay Description Inhibition of human TPMT expressed in Escherichia coli assessed as reduction in SAH level using 6-mercaptopurine as substrate in presence of SAM incu... | J Med Chem 62: 9837-9873 (2019) Article DOI: 10.1021/acs.jmedchem.9b01238 BindingDB Entry DOI: 10.7270/Q2D50RDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiopurine S-methyltransferase (Homo sapiens (Human)) | BDBM50530712 (CHEMBL4591248) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd. Curated by ChEMBL | Assay Description Inhibition of human TPMT expressed in Escherichia coli assessed as reduction in SAH level using 6-mercaptopurine as substrate in presence of SAM incu... | J Med Chem 62: 9837-9873 (2019) Article DOI: 10.1021/acs.jmedchem.9b01238 BindingDB Entry DOI: 10.7270/Q2D50RDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||