

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM198126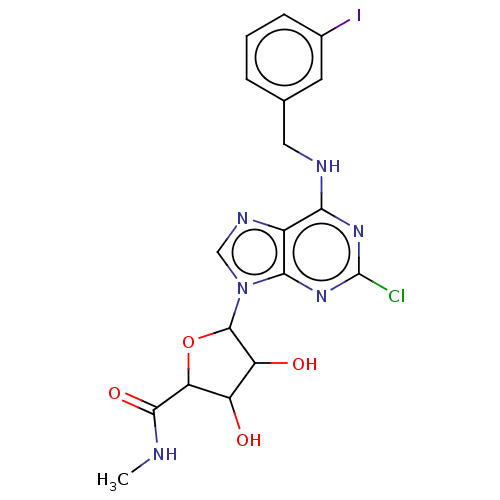 ((2S,3S,4R,5R)-5-[2-chloro-6-[(3-iodophenyl)methyla...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.220 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
UniversitÓ di Napoli Federico II | Assay Description Aliquots of cell membranes (90 ug) were incubated at 25 °C for 180 min in 500 uL of binding buffer (50 mM Tris-HCl, 5 mM MgCl2, 1 mM EDTA, 2 un... | Chem Biol Drug Des 88: 724-729 (2016) Article DOI: 10.1111/cbdd.12801 BindingDB Entry DOI: 10.7270/Q25M64JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM106372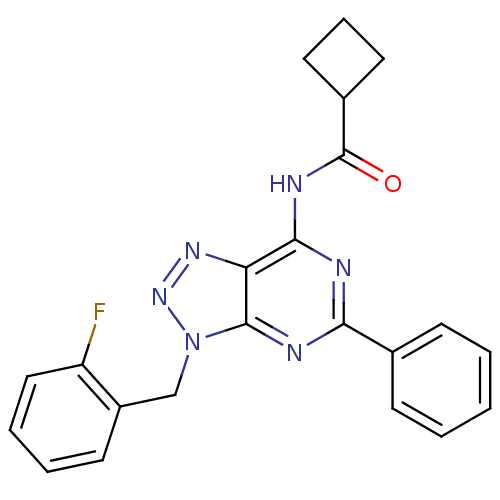 (N-[9-(ortho-fluorobenzyl)-2-phenyl-9H-8-azapurin-6...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Universit£ di Pisa | Assay Description For A1 adenosine receptors, 40 mg of protein were incubated for 60 min at 25°C with [3H]DPCPX 0.5 nM (Kd = 0.4 nM) and increasing concentrations... | Chem Biol Drug Des 82: 22-38 (2013) Article DOI: 10.1111/cbdd.12131 BindingDB Entry DOI: 10.7270/Q2445K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM86761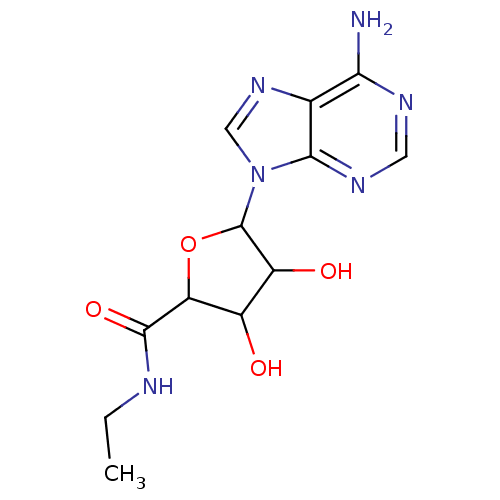 (CAS_35920-39-9 | NECA | NSC_448222) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | Article PubMed | 73 | -40.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
UniversitÓ di Napoli Federico II | Assay Description Aliquots of cell membranes (90 ug) were incubated at 25 °C for 180 min in 500 uL of binding buffer (50 mM Tris-HCl, 5 mM MgCl2, 1 mM EDTA, 2 un... | Chem Biol Drug Des 88: 724-729 (2016) Article DOI: 10.1111/cbdd.12801 BindingDB Entry DOI: 10.7270/Q25M64JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM106376 (N-[9-(ortho-fluorobenzyl)-2-phenyl-9H-8-azapurin-6...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Universit£ di Pisa | Assay Description For A1 adenosine receptors, 40 mg of protein were incubated for 60 min at 25°C with [3H]DPCPX 0.5 nM (Kd = 0.4 nM) and increasing concentrations... | Chem Biol Drug Des 82: 22-38 (2013) Article DOI: 10.1111/cbdd.12131 BindingDB Entry DOI: 10.7270/Q2445K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM106373 (N-[9-(ortho-fluorobenzyl)-2-phenyl-9H-8-azapurin-6...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Universit£ di Pisa | Assay Description For A1 adenosine receptors, 40 mg of protein were incubated for 60 min at 25°C with [3H]DPCPX 0.5 nM (Kd = 0.4 nM) and increasing concentrations... | Chem Biol Drug Des 82: 22-38 (2013) Article DOI: 10.1111/cbdd.12131 BindingDB Entry DOI: 10.7270/Q2445K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM106375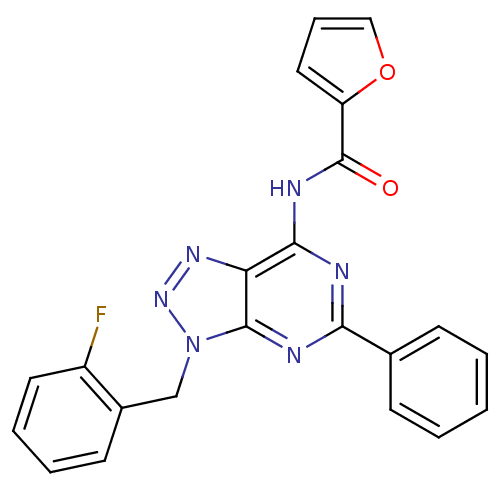 (N-[9-(ortho-fluorobenzyl)-2-phenyl-9H-8-azapurin-6...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 224 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Universit£ di Pisa | Assay Description For A1 adenosine receptors, 40 mg of protein were incubated for 60 min at 25°C with [3H]DPCPX 0.5 nM (Kd = 0.4 nM) and increasing concentrations... | Chem Biol Drug Des 82: 22-38 (2013) Article DOI: 10.1111/cbdd.12131 BindingDB Entry DOI: 10.7270/Q2445K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM106371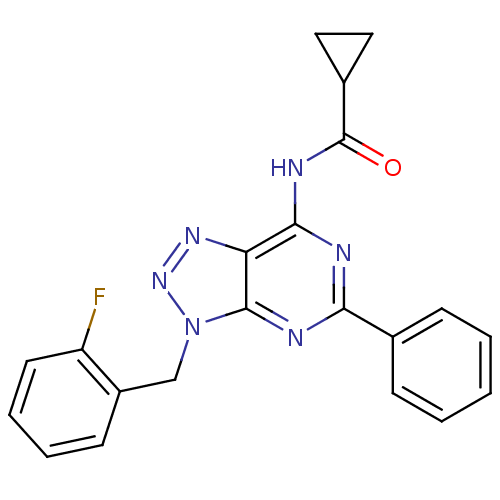 (N-[9-(ortho-fluorobenzyl)-2-phenyl-9H-8-azapurin-6...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 292 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Universit£ di Pisa | Assay Description For A1 adenosine receptors, 40 mg of protein were incubated for 60 min at 25°C with [3H]DPCPX 0.5 nM (Kd = 0.4 nM) and increasing concentrations... | Chem Biol Drug Des 82: 22-38 (2013) Article DOI: 10.1111/cbdd.12131 BindingDB Entry DOI: 10.7270/Q2445K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM106374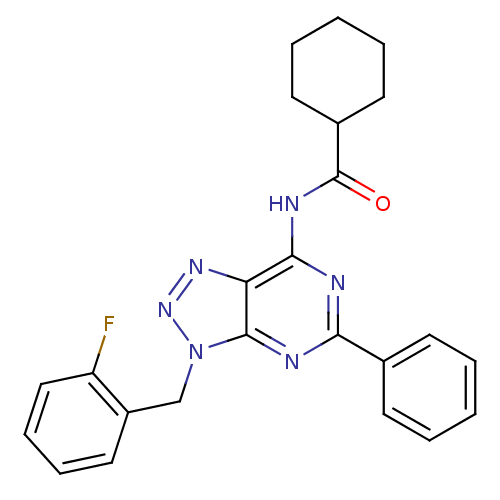 (N-[9-(ortho-fluorobenzyl)-2-phenyl-9H-8-azapurin-6...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 413 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Universit£ di Pisa | Assay Description For A1 adenosine receptors, 40 mg of protein were incubated for 60 min at 25°C with [3H]DPCPX 0.5 nM (Kd = 0.4 nM) and increasing concentrations... | Chem Biol Drug Des 82: 22-38 (2013) Article DOI: 10.1111/cbdd.12131 BindingDB Entry DOI: 10.7270/Q2445K4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM198128 (4-amino-6-alkyloxy-2-benzylthiopyrimidines (5)) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | >-28.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
UniversitÓ di Napoli Federico II | Assay Description Aliquots of cell membranes (90 ug) were incubated at 25 °C for 180 min in 500 uL of binding buffer (50 mM Tris-HCl, 5 mM MgCl2, 1 mM EDTA, 2 un... | Chem Biol Drug Des 88: 724-729 (2016) Article DOI: 10.1111/cbdd.12801 BindingDB Entry DOI: 10.7270/Q25M64JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | >-28.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
UniversitÓ di Napoli Federico II | Assay Description Aliquots of cell membranes (90 ug) were incubated at 25 °C for 180 min in 500 uL of binding buffer (50 mM Tris-HCl, 5 mM MgCl2, 1 mM EDTA, 2 un... | Chem Biol Drug Des 88: 724-729 (2016) Article DOI: 10.1111/cbdd.12801 BindingDB Entry DOI: 10.7270/Q25M64JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM198127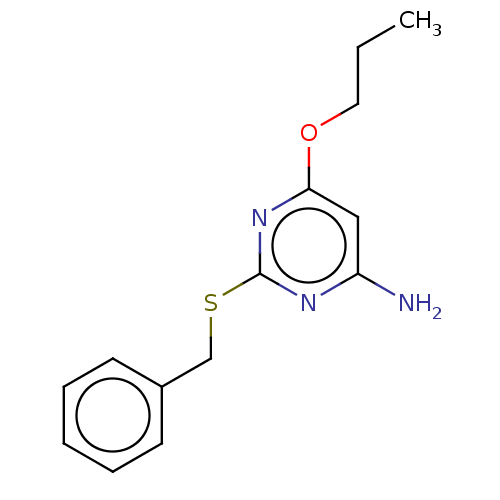 (4-amino-6-alkyloxy-2-benzylthiopyrimidines (4)) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | >-28.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
UniversitÓ di Napoli Federico II | Assay Description Aliquots of cell membranes (90 ug) were incubated at 25 °C for 180 min in 500 uL of binding buffer (50 mM Tris-HCl, 5 mM MgCl2, 1 mM EDTA, 2 un... | Chem Biol Drug Des 88: 724-729 (2016) Article DOI: 10.1111/cbdd.12801 BindingDB Entry DOI: 10.7270/Q25M64JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM198129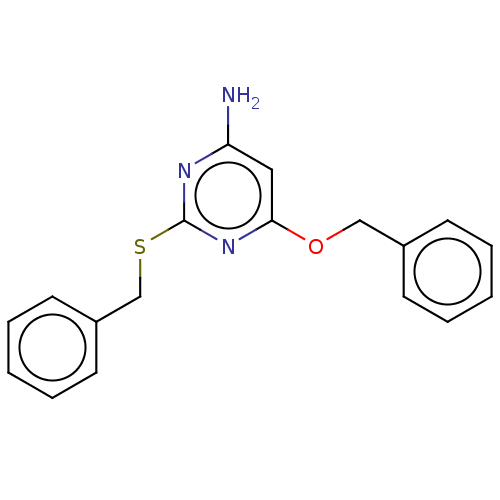 (4-amino-6-alkyloxy-2-benzylthiopyrimidines (6)) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | >-28.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
UniversitÓ di Napoli Federico II | Assay Description Aliquots of cell membranes (90 ug) were incubated at 25 °C for 180 min in 500 uL of binding buffer (50 mM Tris-HCl, 5 mM MgCl2, 1 mM EDTA, 2 un... | Chem Biol Drug Des 88: 724-729 (2016) Article DOI: 10.1111/cbdd.12801 BindingDB Entry DOI: 10.7270/Q25M64JG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM214690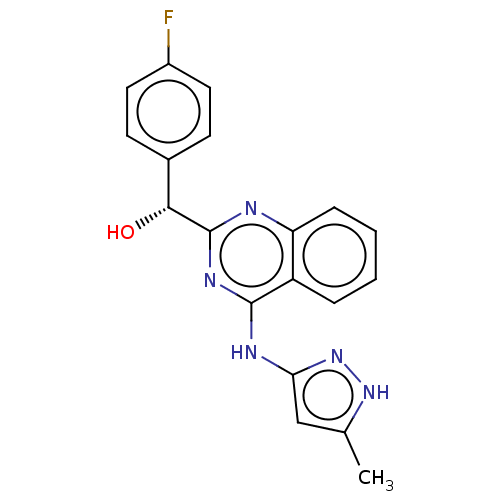 (US9295672, (R)-(4-fluorophenyl)(4-((5-methyl-1H-py...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Ambit Biosciences Corporation US Patent | Assay Description .(R)- and (S)-(4-Fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol was tested in a radioligand binding assay, according to th... | US Patent US9295672 (2016) BindingDB Entry DOI: 10.7270/Q2ZP44ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM214690 (US9295672, (R)-(4-fluorophenyl)(4-((5-methyl-1H-py...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 28.5 | n/a | n/a | n/a | 7.4 | 25 |
Ambit Biosciences Corporation US Patent | Assay Description .(R)- and (S)-(4-Fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol was tested in a radioligand binding assay, according to th... | US Patent US9295672 (2016) BindingDB Entry DOI: 10.7270/Q2ZP44ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM214689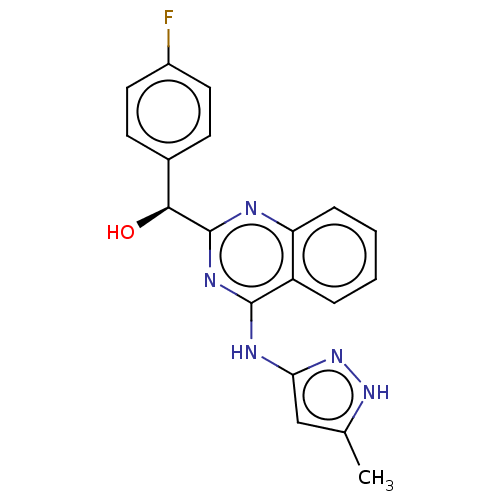 (US9295672, (S)-(4-fluorophenyl)(4-((5-methyl-1H-py...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 1.42 | n/a | n/a | n/a | 7.4 | 25 |
Ambit Biosciences Corporation US Patent | Assay Description .(R)- and (S)-(4-Fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol was tested in a radioligand binding assay, according to th... | US Patent US9295672 (2016) BindingDB Entry DOI: 10.7270/Q2ZP44ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM214689 (US9295672, (S)-(4-fluorophenyl)(4-((5-methyl-1H-py...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1.55 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Ambit Biosciences Corporation US Patent | Assay Description .(R)- and (S)-(4-Fluorophenyl)(4-((5-methyl-1H-pyrazol-3-yl)amino)quinazolin-2-yl)methanol was tested in a radioligand binding assay, according to th... | US Patent US9295672 (2016) BindingDB Entry DOI: 10.7270/Q2ZP44ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 [I248L] (Homo sapiens (Human)) | BDBM191046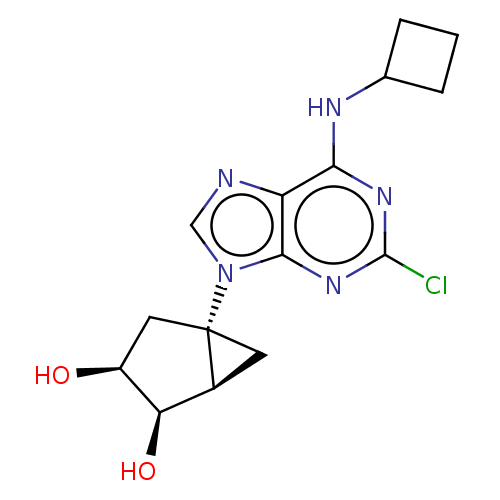 (US9181253, 109) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5.90 | -47.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
The United States of America, as represented by the Secretary, Department of Health and Human Services US Patent | Assay Description Radioligand binding assays at A1, A2A, and A3ARs were performed according to the procedures described previously. Each tube in the binding assay cont... | US Patent US9181253 (2015) BindingDB Entry DOI: 10.7270/Q2CV4GJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 [I248L] (Homo sapiens (Human)) | BDBM191068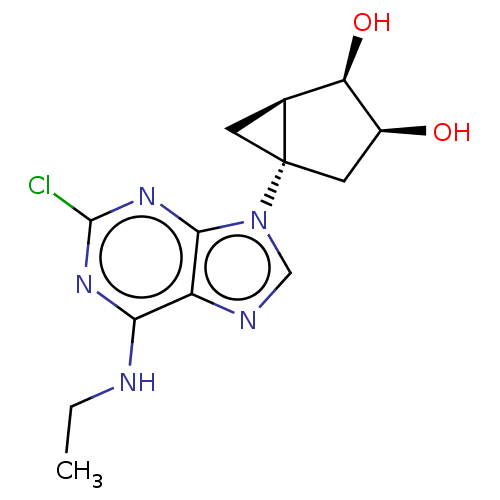 (US9181253, 115) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 6.60 | -46.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
The United States of America, as represented by the Secretary, Department of Health and Human Services US Patent | Assay Description Radioligand binding assays at A1, A2A, and A3ARs were performed according to the procedures described previously. Each tube in the binding assay cont... | US Patent US9181253 (2015) BindingDB Entry DOI: 10.7270/Q2CV4GJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 [I248L] (Homo sapiens (Human)) | BDBM191057 (US9181253, 119) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 8.90 | -46.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
The United States of America, as represented by the Secretary, Department of Health and Human Services US Patent | Assay Description Radioligand binding assays at A1, A2A, and A3ARs were performed according to the procedures described previously. Each tube in the binding assay cont... | US Patent US9181253 (2015) BindingDB Entry DOI: 10.7270/Q2CV4GJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 [I248L] (Homo sapiens (Human)) | BDBM191061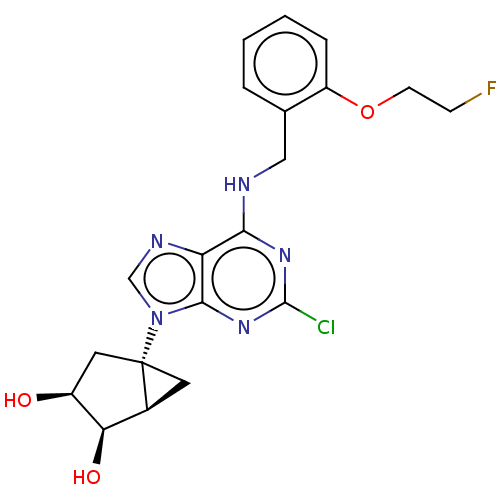 (US9181253, 123) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 10.3 | -45.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
The United States of America, as represented by the Secretary, Department of Health and Human Services US Patent | Assay Description Radioligand binding assays at A1, A2A, and A3ARs were performed according to the procedures described previously. Each tube in the binding assay cont... | US Patent US9181253 (2015) BindingDB Entry DOI: 10.7270/Q2CV4GJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 [I248L] (Homo sapiens (Human)) | BDBM191055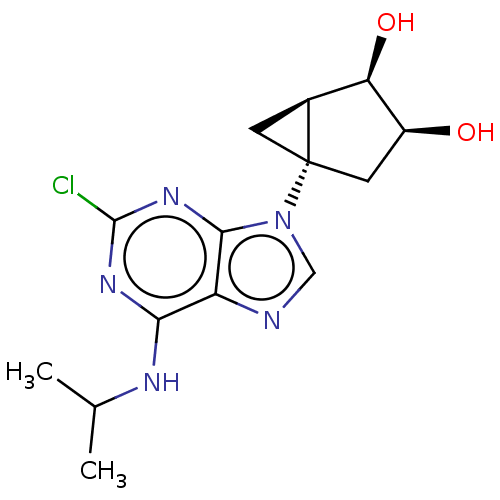 (US9181253, 117) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 12 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
The United States of America, as represented by the Secretary, Department of Health and Human Services US Patent | Assay Description Radioligand binding assays at A1, A2A, and A3ARs were performed according to the procedures described previously. Each tube in the binding assay cont... | US Patent US9181253 (2015) BindingDB Entry DOI: 10.7270/Q2CV4GJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 [I248L] (Homo sapiens (Human)) | BDBM191045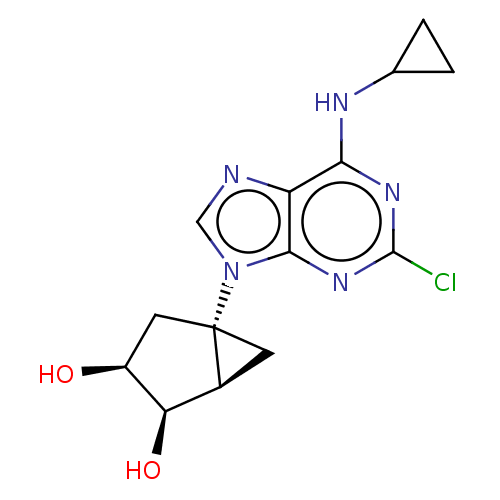 (US9181253, 108) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 12.1 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
The United States of America, as represented by the Secretary, Department of Health and Human Services US Patent | Assay Description Radioligand binding assays at A1, A2A, and A3ARs were performed according to the procedures described previously. Each tube in the binding assay cont... | US Patent US9181253 (2015) BindingDB Entry DOI: 10.7270/Q2CV4GJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 [I248L] (Homo sapiens (Human)) | BDBM191062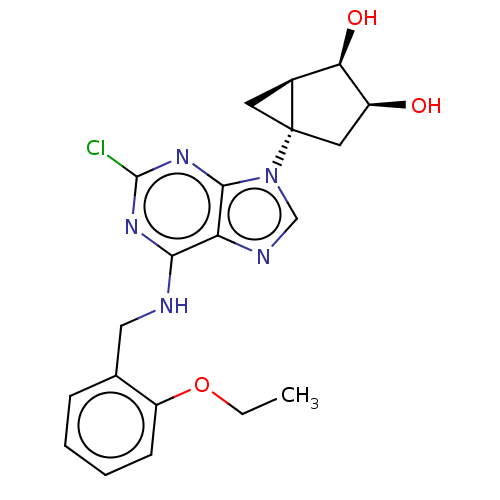 (US9181253, 124) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 15.2 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
The United States of America, as represented by the Secretary, Department of Health and Human Services US Patent | Assay Description Radioligand binding assays at A1, A2A, and A3ARs were performed according to the procedures described previously. Each tube in the binding assay cont... | US Patent US9181253 (2015) BindingDB Entry DOI: 10.7270/Q2CV4GJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 [I248L] (Homo sapiens (Human)) | BDBM191064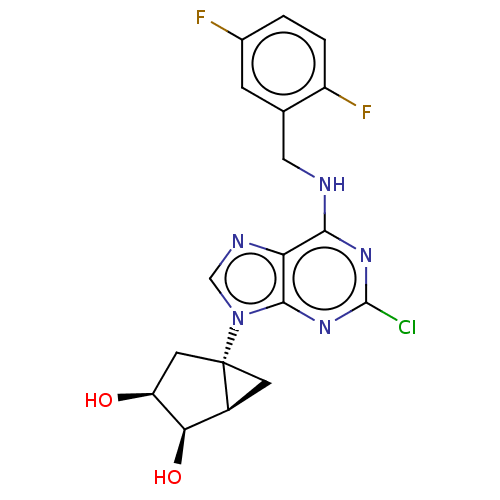 (US9181253, 126) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 16.5 | -44.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
The United States of America, as represented by the Secretary, Department of Health and Human Services US Patent | Assay Description Radioligand binding assays at A1, A2A, and A3ARs were performed according to the procedures described previously. Each tube in the binding assay cont... | US Patent US9181253 (2015) BindingDB Entry DOI: 10.7270/Q2CV4GJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 [I248L] (Homo sapiens (Human)) | BDBM191069 (US9181253, 116) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 32.4 | -42.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
The United States of America, as represented by the Secretary, Department of Health and Human Services US Patent | Assay Description Radioligand binding assays at A1, A2A, and A3ARs were performed according to the procedures described previously. Each tube in the binding assay cont... | US Patent US9181253 (2015) BindingDB Entry DOI: 10.7270/Q2CV4GJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 [I248L] (Homo sapiens (Human)) | BDBM191066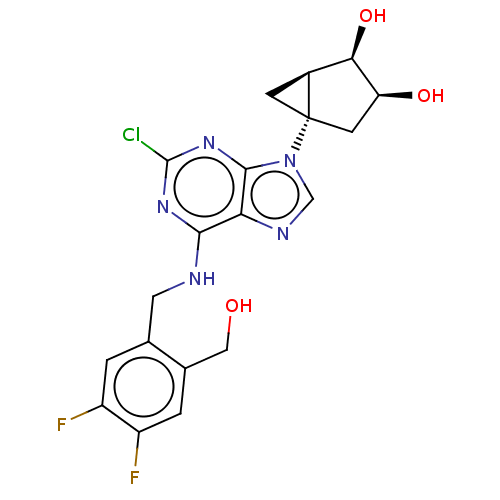 (US9181253, 128) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 40.4 | -42.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
The United States of America, as represented by the Secretary, Department of Health and Human Services US Patent | Assay Description Radioligand binding assays at A1, A2A, and A3ARs were performed according to the procedures described previously. Each tube in the binding assay cont... | US Patent US9181253 (2015) BindingDB Entry DOI: 10.7270/Q2CV4GJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 [I248L] (Homo sapiens (Human)) | BDBM191060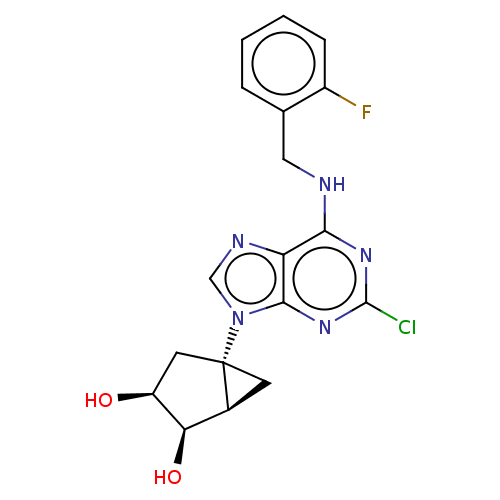 (US9181253, 122) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 41.5 | -42.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
The United States of America, as represented by the Secretary, Department of Health and Human Services US Patent | Assay Description Radioligand binding assays at A1, A2A, and A3ARs were performed according to the procedures described previously. Each tube in the binding assay cont... | US Patent US9181253 (2015) BindingDB Entry DOI: 10.7270/Q2CV4GJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 [I248L] (Homo sapiens (Human)) | BDBM191056 (US9181253, 118) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 52 | -41.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
The United States of America, as represented by the Secretary, Department of Health and Human Services US Patent | Assay Description Radioligand binding assays at A1, A2A, and A3ARs were performed according to the procedures described previously. Each tube in the binding assay cont... | US Patent US9181253 (2015) BindingDB Entry DOI: 10.7270/Q2CV4GJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 [I248L] (Homo sapiens (Human)) | BDBM191065 (US9181253, 127) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 83.2 | -40.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
The United States of America, as represented by the Secretary, Department of Health and Human Services US Patent | Assay Description Radioligand binding assays at A1, A2A, and A3ARs were performed according to the procedures described previously. Each tube in the binding assay cont... | US Patent US9181253 (2015) BindingDB Entry DOI: 10.7270/Q2CV4GJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 [I248L] (Homo sapiens (Human)) | BDBM191058 (US9181253, 120c) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 110 | -39.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
The United States of America, as represented by the Secretary, Department of Health and Human Services US Patent | Assay Description Radioligand binding assays at A1, A2A, and A3ARs were performed according to the procedures described previously. Each tube in the binding assay cont... | US Patent US9181253 (2015) BindingDB Entry DOI: 10.7270/Q2CV4GJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 [I248L] (Homo sapiens (Human)) | BDBM191063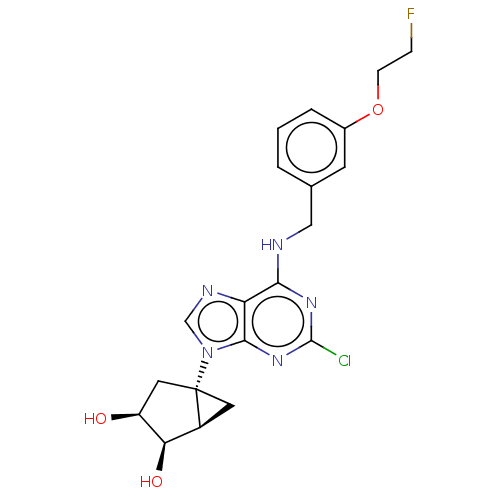 (US9181253, 125) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 114 | -39.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
The United States of America, as represented by the Secretary, Department of Health and Human Services US Patent | Assay Description Radioligand binding assays at A1, A2A, and A3ARs were performed according to the procedures described previously. Each tube in the binding assay cont... | US Patent US9181253 (2015) BindingDB Entry DOI: 10.7270/Q2CV4GJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 [I248L] (Homo sapiens (Human)) | BDBM191067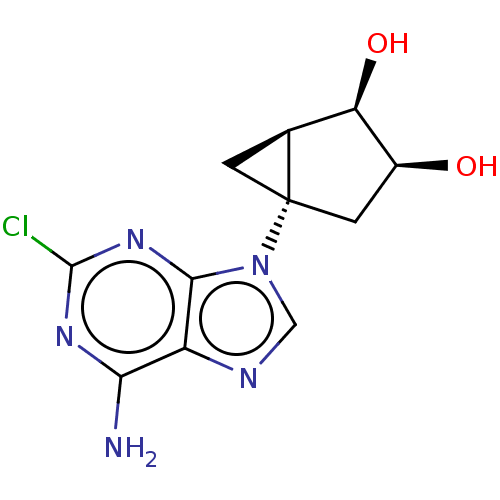 (US9181253, 107) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 160 | -38.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
The United States of America, as represented by the Secretary, Department of Health and Human Services US Patent | Assay Description Radioligand binding assays at A1, A2A, and A3ARs were performed according to the procedures described previously. Each tube in the binding assay cont... | US Patent US9181253 (2015) BindingDB Entry DOI: 10.7270/Q2CV4GJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 [I248L] (Homo sapiens (Human)) | BDBM191054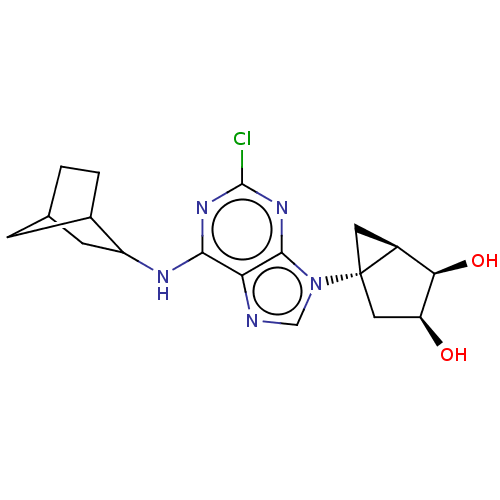 (US9181253, 114) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 236 | -37.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
The United States of America, as represented by the Secretary, Department of Health and Human Services US Patent | Assay Description Radioligand binding assays at A1, A2A, and A3ARs were performed according to the procedures described previously. Each tube in the binding assay cont... | US Patent US9181253 (2015) BindingDB Entry DOI: 10.7270/Q2CV4GJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 [I248L] (Homo sapiens (Human)) | BDBM191053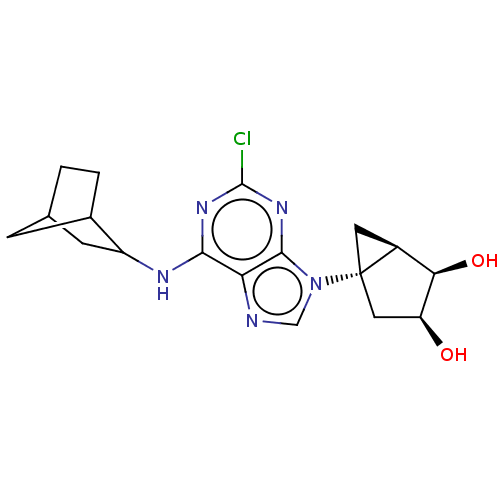 (US9181253, 113) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 315 | -37.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
The United States of America, as represented by the Secretary, Department of Health and Human Services US Patent | Assay Description Radioligand binding assays at A1, A2A, and A3ARs were performed according to the procedures described previously. Each tube in the binding assay cont... | US Patent US9181253 (2015) BindingDB Entry DOI: 10.7270/Q2CV4GJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 [I248L] (Homo sapiens (Human)) | BDBM191059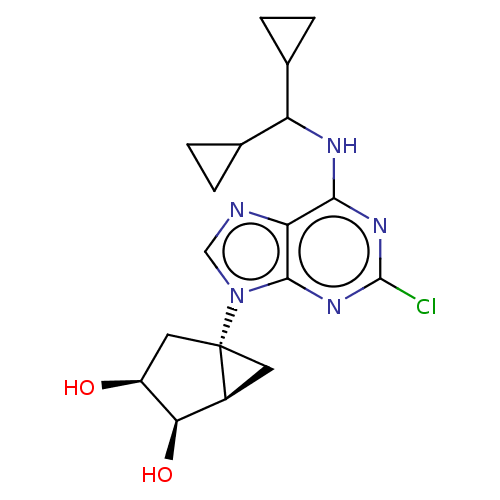 (US9181253, 121) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 470 | -36.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
The United States of America, as represented by the Secretary, Department of Health and Human Services US Patent | Assay Description Radioligand binding assays at A1, A2A, and A3ARs were performed according to the procedures described previously. Each tube in the binding assay cont... | US Patent US9181253 (2015) BindingDB Entry DOI: 10.7270/Q2CV4GJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 [I248L] (Homo sapiens (Human)) | BDBM191050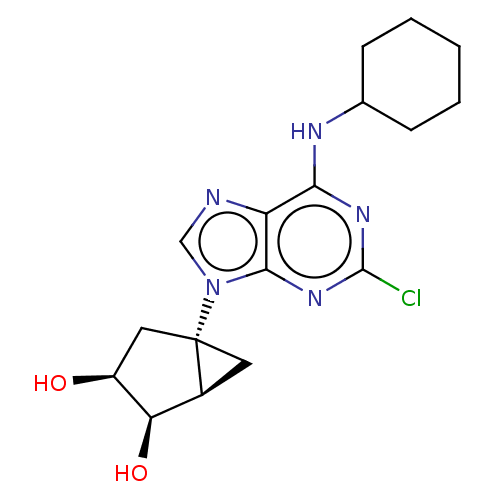 (US9181253, 110) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 500 | -36.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
The United States of America, as represented by the Secretary, Department of Health and Human Services US Patent | Assay Description Radioligand binding assays at A1, A2A, and A3ARs were performed according to the procedures described previously. Each tube in the binding assay cont... | US Patent US9181253 (2015) BindingDB Entry DOI: 10.7270/Q2CV4GJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 [I248L] (Homo sapiens (Human)) | BDBM191051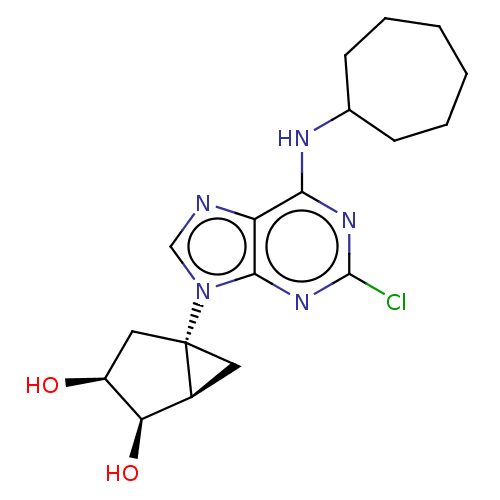 (US9181253, 111) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 560 | -35.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
The United States of America, as represented by the Secretary, Department of Health and Human Services US Patent | Assay Description Radioligand binding assays at A1, A2A, and A3ARs were performed according to the procedures described previously. Each tube in the binding assay cont... | US Patent US9181253 (2015) BindingDB Entry DOI: 10.7270/Q2CV4GJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 [I248L] (Homo sapiens (Human)) | BDBM191052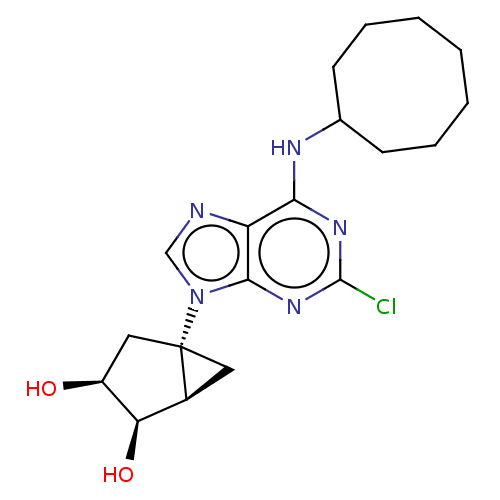 (US9181253, 112) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.53E+3 | -33.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
The United States of America, as represented by the Secretary, Department of Health and Human Services US Patent | Assay Description Radioligand binding assays at A1, A2A, and A3ARs were performed according to the procedures described previously. Each tube in the binding assay cont... | US Patent US9181253 (2015) BindingDB Entry DOI: 10.7270/Q2CV4GJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM224619 (US9326978, 32) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 410 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
The United States of America, Represented by the Secretary, Dept. of Health and Human Services; Universiteit Leiden US Patent | Assay Description Each tube in the competitive binding assay contained 100 μl membrane suspension (20 μg protein), 50 μl [125I]-AB-MECA (0.5 nM), and 50... | US Patent US9326978 (2016) BindingDB Entry DOI: 10.7270/Q2C24V85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM224617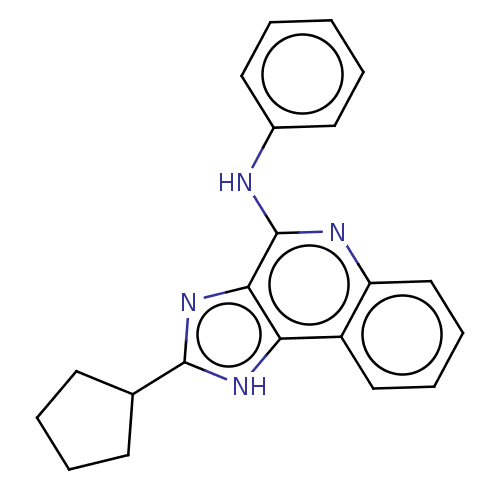 (US9326978, 30) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 786 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
The United States of America, Represented by the Secretary, Dept. of Health and Human Services; Universiteit Leiden US Patent | Assay Description Each tube in the competitive binding assay contained 100 μl membrane suspension (20 μg protein), 50 μl [125I]-AB-MECA (0.5 nM), and 50... | US Patent US9326978 (2016) BindingDB Entry DOI: 10.7270/Q2C24V85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM224618 (US9326978, 31) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
The United States of America, Represented by the Secretary, Dept. of Health and Human Services; Universiteit Leiden US Patent | Assay Description Each tube in the competitive binding assay contained 100 μl membrane suspension (20 μg protein), 50 μl [125I]-AB-MECA (0.5 nM), and 50... | US Patent US9326978 (2016) BindingDB Entry DOI: 10.7270/Q2C24V85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM224621 (US9326978, 34) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
The United States of America, Represented by the Secretary, Dept. of Health and Human Services; Universiteit Leiden US Patent | Assay Description Each tube in the competitive binding assay contained 100 μl membrane suspension (20 μg protein), 50 μl [125I]-AB-MECA (0.5 nM), and 50... | US Patent US9326978 (2016) BindingDB Entry DOI: 10.7270/Q2C24V85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM224620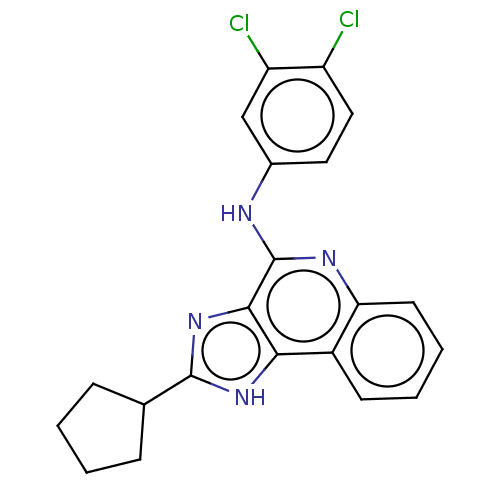 (US9326978, 33 | US9326978, 49) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
The United States of America, Represented by the Secretary, Dept. of Health and Human Services; Universiteit Leiden US Patent | Assay Description Each tube in the competitive binding assay contained 100 μl membrane suspension (20 μg protein), 50 μl [125I]-AB-MECA (0.5 nM), and 50... | US Patent US9326978 (2016) BindingDB Entry DOI: 10.7270/Q2C24V85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM224620 (US9326978, 33 | US9326978, 49) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
The United States of America, Represented by the Secretary, Dept. of Health and Human Services; Universiteit Leiden US Patent | Assay Description Each tube in the competitive binding assay contained 100 μl membrane suspension (20 μg protein), 50 μl [125I]-AB-MECA (0.5 nM), and 50... | US Patent US9326978 (2016) BindingDB Entry DOI: 10.7270/Q2C24V85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50059414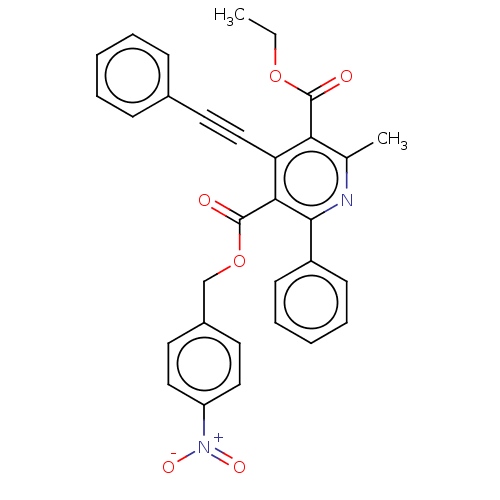 (2-Methyl-6-phenyl-4-phenylethynyl-1,4-dihydro-pyri...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity at cloned human adenosine A3 receptor expressed in HEK-293 cells was determined using [125I]-AB-MECA as radioligand | J Med Chem 41: 2835-45 (1998) Article DOI: 10.1021/jm980094b BindingDB Entry DOI: 10.7270/Q25Q4V7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50117108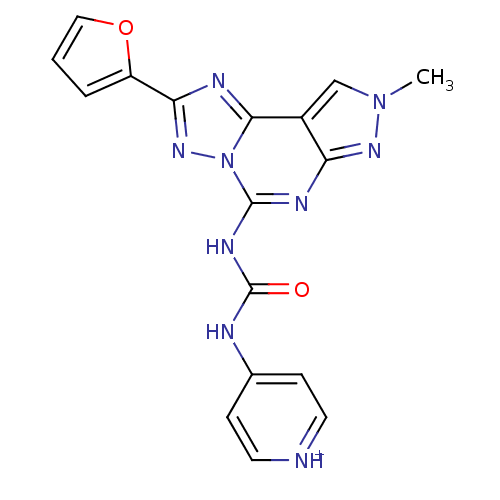 (4-[3-(2-Furan-2-yl-8-methyl-8H-pyrazolo[4,3-e][1,2...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-MRE 3008-F20 from Human Adenosine A3 receptor expressed in HEK-293 cells | J Med Chem 45: 3579-82 (2002) BindingDB Entry DOI: 10.7270/Q2SN08B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50343133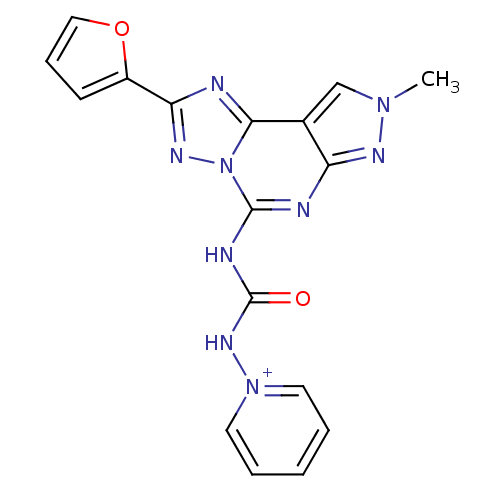 (1-(3-(2-(furan-2-yl)-8-methyl-8H-pyrazolo[4,3-e][1...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Singapore Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A3 receptor | Bioorg Med Chem Lett 21: 2898-905 (2011) Article DOI: 10.1016/j.bmcl.2011.03.073 BindingDB Entry DOI: 10.7270/Q2VH5P5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50117109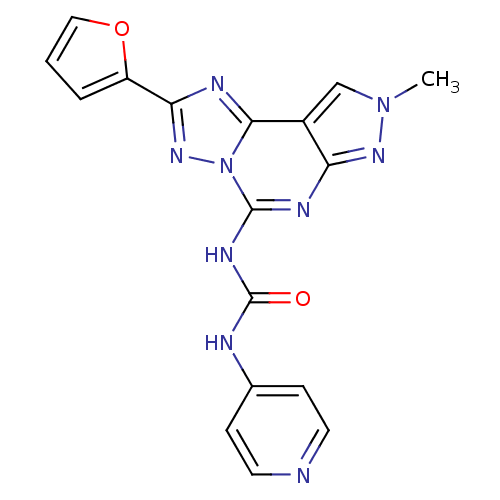 (1-(2-Furan-2-yl-8-methyl-8H-pyrazolo[4,3-e][1,2,4]...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human recombinant adenosine A3 receptor expressed in CHO cells after 60 mins by gamma counter | J Med Chem 55: 5676-703 (2012) Article DOI: 10.1021/jm300087j BindingDB Entry DOI: 10.7270/Q25H7HDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50117108 (4-[3-(2-Furan-2-yl-8-methyl-8H-pyrazolo[4,3-e][1,2...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Displacement of [3H]MRE-3008-F20 from human adenosine A3 receptor expressed in CHO cells after 120 mins by scintillation counter | J Med Chem 55: 5380-90 (2012) Article DOI: 10.1021/jm300323t BindingDB Entry DOI: 10.7270/Q2CN7509 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50117108 (4-[3-(2-Furan-2-yl-8-methyl-8H-pyrazolo[4,3-e][1,2...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Trieste Curated by ChEMBL | Assay Description Displacement of [3H]MRE3008-F20 from human adenosine A3 receptor expressed in HEK293 cells | J Med Chem 49: 1720-9 (2006) Article DOI: 10.1021/jm051147+ BindingDB Entry DOI: 10.7270/Q26974CT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 8744 total ) | Next | Last >> |