

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ephrin type-A receptor 4 [29-209,M164A] (Homo sapiens (Human)) | BDBM223311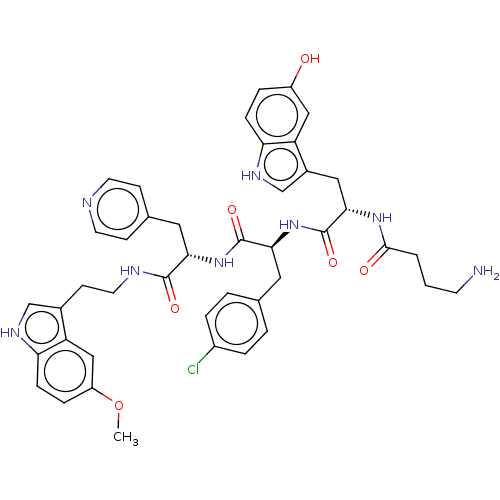 (123C4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 62 | n/a | n/a | n/a | 6.5 | 27 |
Sanford-Burnham-Prebys Medical Discovery Institute | Assay Description The purified EphA proteins (EphA4-LBD, EphA3-LBD, and EphA2-LBD) were dissolved in 50 mM potassium phosphate buffer (pH 6.5) containing 100 mM NaCl. ... | Cell Chem Biol 24: 293-305 (2017) Article DOI: 10.1016/j.chembiol.2017.01.006 BindingDB Entry DOI: 10.7270/Q2GX49FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 [29-209,I59A] (Homo sapiens (Human)) | BDBM223311 (123C4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 9.09E+3 | n/a | n/a | n/a | 6.5 | 27 |
Sanford-Burnham-Prebys Medical Discovery Institute | Assay Description The purified EphA proteins (EphA4-LBD, EphA3-LBD, and EphA2-LBD) were dissolved in 50 mM potassium phosphate buffer (pH 6.5) containing 100 mM NaCl. ... | Cell Chem Biol 24: 293-305 (2017) Article DOI: 10.1016/j.chembiol.2017.01.006 BindingDB Entry DOI: 10.7270/Q2GX49FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 [29-209,I159A] (Homo sapiens (Human)) | BDBM223311 (123C4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 290 | n/a | n/a | n/a | 6.5 | 27 |
Sanford-Burnham-Prebys Medical Discovery Institute | Assay Description The purified EphA proteins (EphA4-LBD, EphA3-LBD, and EphA2-LBD) were dissolved in 50 mM potassium phosphate buffer (pH 6.5) containing 100 mM NaCl. ... | Cell Chem Biol 24: 293-305 (2017) Article DOI: 10.1016/j.chembiol.2017.01.006 BindingDB Entry DOI: 10.7270/Q2GX49FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 [29-209,I67A] (Homo sapiens (Human)) | BDBM223311 (123C4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 780 | n/a | n/a | n/a | 6.5 | 27 |
Sanford-Burnham-Prebys Medical Discovery Institute | Assay Description The purified EphA proteins (EphA4-LBD, EphA3-LBD, and EphA2-LBD) were dissolved in 50 mM potassium phosphate buffer (pH 6.5) containing 100 mM NaCl. ... | Cell Chem Biol 24: 293-305 (2017) Article DOI: 10.1016/j.chembiol.2017.01.006 BindingDB Entry DOI: 10.7270/Q2GX49FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 [29-209,D61G,E62G] (Homo sapiens (Human)) | BDBM223311 (123C4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 340 | n/a | n/a | n/a | 6.5 | 27 |
Sanford-Burnham-Prebys Medical Discovery Institute | Assay Description The purified EphA proteins (EphA4-LBD, EphA3-LBD, and EphA2-LBD) were dissolved in 50 mM potassium phosphate buffer (pH 6.5) containing 100 mM NaCl. ... | Cell Chem Biol 24: 293-305 (2017) Article DOI: 10.1016/j.chembiol.2017.01.006 BindingDB Entry DOI: 10.7270/Q2GX49FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 [29-209,M60A] (Homo sapiens (Human)) | BDBM223311 (123C4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 350 | n/a | n/a | n/a | 6.5 | 27 |
Sanford-Burnham-Prebys Medical Discovery Institute | Assay Description The purified EphA proteins (EphA4-LBD, EphA3-LBD, and EphA2-LBD) were dissolved in 50 mM potassium phosphate buffer (pH 6.5) containing 100 mM NaCl. ... | Cell Chem Biol 24: 293-305 (2017) Article DOI: 10.1016/j.chembiol.2017.01.006 BindingDB Entry DOI: 10.7270/Q2GX49FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 [29-209,I59G] (Homo sapiens (Human)) | BDBM223311 (123C4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 3.66E+3 | n/a | n/a | n/a | 6.5 | 27 |
Sanford-Burnham-Prebys Medical Discovery Institute | Assay Description The purified EphA proteins (EphA4-LBD, EphA3-LBD, and EphA2-LBD) were dissolved in 50 mM potassium phosphate buffer (pH 6.5) containing 100 mM NaCl. ... | Cell Chem Biol 24: 293-305 (2017) Article DOI: 10.1016/j.chembiol.2017.01.006 BindingDB Entry DOI: 10.7270/Q2GX49FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 [29-209] (Homo sapiens (Human)) | BDBM223311 (123C4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 420 | n/a | n/a | n/a | 6.5 | 27 |
Sanford-Burnham-Prebys Medical Discovery Institute | Assay Description The purified EphA proteins (EphA4-LBD, EphA3-LBD, and EphA2-LBD) were dissolved in 50 mM potassium phosphate buffer (pH 6.5) containing 100 mM NaCl. ... | Cell Chem Biol 24: 293-305 (2017) Article DOI: 10.1016/j.chembiol.2017.01.006 BindingDB Entry DOI: 10.7270/Q2GX49FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 [29-209] (Homo sapiens (Human)) | BDBM223310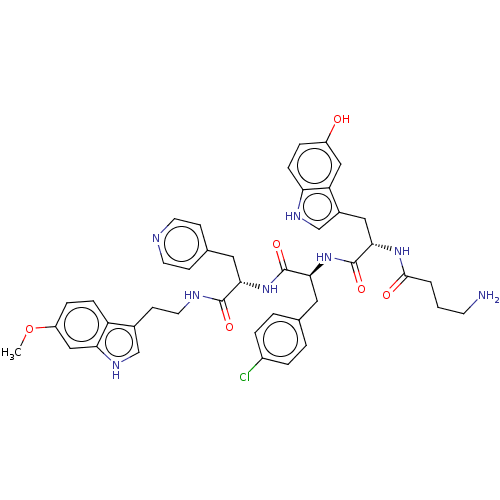 (123C7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 540 | n/a | n/a | n/a | 6.5 | 27 |
Sanford-Burnham-Prebys Medical Discovery Institute | Assay Description The purified EphA proteins (EphA4-LBD, EphA3-LBD, and EphA2-LBD) were dissolved in 50 mM potassium phosphate buffer (pH 6.5) containing 100 mM NaCl. ... | Cell Chem Biol 24: 293-305 (2017) Article DOI: 10.1016/j.chembiol.2017.01.006 BindingDB Entry DOI: 10.7270/Q2GX49FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 [29-209] (Homo sapiens (Human)) | BDBM223308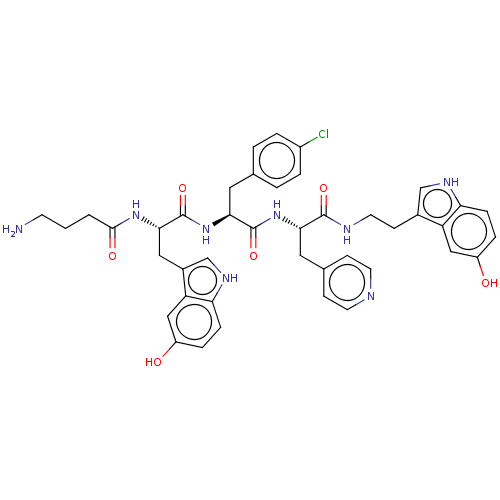 (123C5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | 6.5 | 27 |
Sanford-Burnham-Prebys Medical Discovery Institute | Assay Description The purified EphA proteins (EphA4-LBD, EphA3-LBD, and EphA2-LBD) were dissolved in 50 mM potassium phosphate buffer (pH 6.5) containing 100 mM NaCl. ... | Cell Chem Biol 24: 293-305 (2017) Article DOI: 10.1016/j.chembiol.2017.01.006 BindingDB Entry DOI: 10.7270/Q2GX49FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 [29-209] (Homo sapiens (Human)) | BDBM223305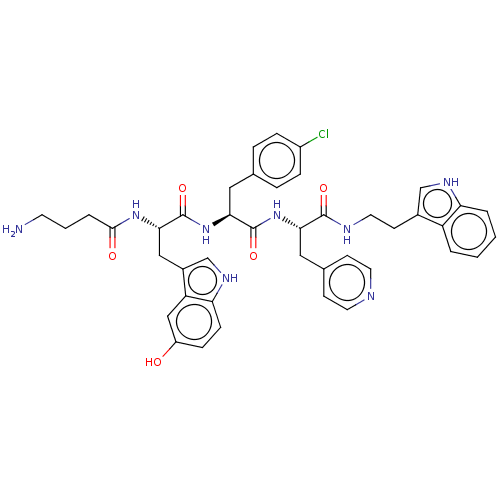 (123C2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | 6.5 | 27 |
Sanford-Burnham-Prebys Medical Discovery Institute | Assay Description The purified EphA proteins (EphA4-LBD, EphA3-LBD, and EphA2-LBD) were dissolved in 50 mM potassium phosphate buffer (pH 6.5) containing 100 mM NaCl. ... | Cell Chem Biol 24: 293-305 (2017) Article DOI: 10.1016/j.chembiol.2017.01.006 BindingDB Entry DOI: 10.7270/Q2GX49FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 [29-209] (Homo sapiens (Human)) | BDBM223300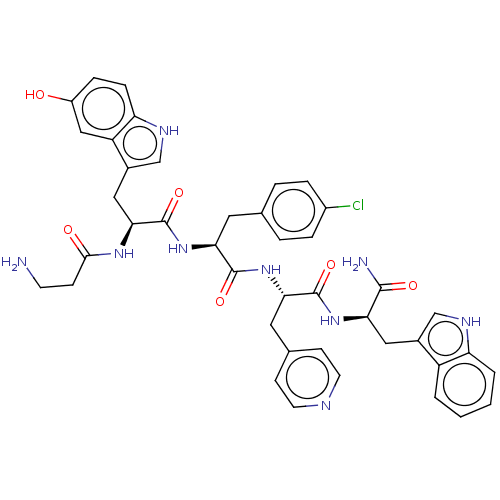 (EphA4-LBC inhibitor, Compound 22) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | 6.5 | 27 |
Sanford-Burnham-Prebys Medical Discovery Institute | Assay Description The purified EphA proteins (EphA4-LBD, EphA3-LBD, and EphA2-LBD) were dissolved in 50 mM potassium phosphate buffer (pH 6.5) containing 100 mM NaCl. ... | Cell Chem Biol 24: 293-305 (2017) Article DOI: 10.1016/j.chembiol.2017.01.006 BindingDB Entry DOI: 10.7270/Q2GX49FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 [29-209] (Homo sapiens (Human)) | BDBM223311 (123C4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 640 | -35.4 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Sanford-Burnham-Prebys Medical Discovery Institute | Assay Description For competitive binding assays, 1 mL of 200 mM EphA4-LBD was pre-incubated with the tested compounds at various concentrations in 98 mL of PBS (pH 7.... | Cell Chem Biol 24: 293-305 (2017) Article DOI: 10.1016/j.chembiol.2017.01.006 BindingDB Entry DOI: 10.7270/Q2GX49FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 [29-209] (Homo sapiens (Human)) | BDBM223301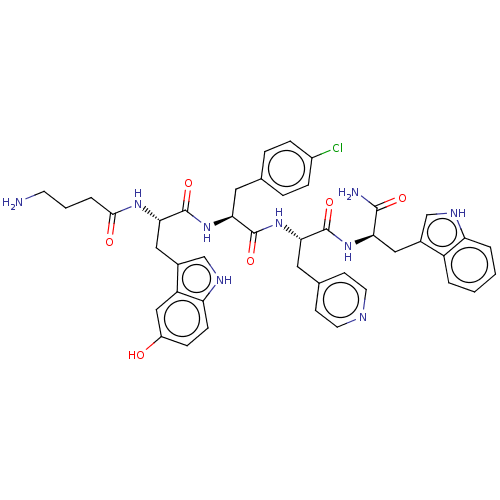 (120H10) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 960 | -34.3 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Sanford-Burnham-Prebys Medical Discovery Institute | Assay Description For competitive binding assays, 1 mL of 200 mM EphA4-LBD was pre-incubated with the tested compounds at various concentrations in 98 mL of PBS (pH 7.... | Cell Chem Biol 24: 293-305 (2017) Article DOI: 10.1016/j.chembiol.2017.01.006 BindingDB Entry DOI: 10.7270/Q2GX49FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 [29-209] (Homo sapiens (Human)) | BDBM223310 (123C7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.59E+3 | -33.1 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Sanford-Burnham-Prebys Medical Discovery Institute | Assay Description For competitive binding assays, 1 mL of 200 mM EphA4-LBD was pre-incubated with the tested compounds at various concentrations in 98 mL of PBS (pH 7.... | Cell Chem Biol 24: 293-305 (2017) Article DOI: 10.1016/j.chembiol.2017.01.006 BindingDB Entry DOI: 10.7270/Q2GX49FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 [29-209] (Homo sapiens (Human)) | BDBM223305 (123C2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.59E+3 | -33.1 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Sanford-Burnham-Prebys Medical Discovery Institute | Assay Description For competitive binding assays, 1 mL of 200 mM EphA4-LBD was pre-incubated with the tested compounds at various concentrations in 98 mL of PBS (pH 7.... | Cell Chem Biol 24: 293-305 (2017) Article DOI: 10.1016/j.chembiol.2017.01.006 BindingDB Entry DOI: 10.7270/Q2GX49FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 [29-209] (Homo sapiens (Human)) | BDBM223300 (EphA4-LBC inhibitor, Compound 22) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.14E+3 | -32.4 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Sanford-Burnham-Prebys Medical Discovery Institute | Assay Description For competitive binding assays, 1 mL of 200 mM EphA4-LBD was pre-incubated with the tested compounds at various concentrations in 98 mL of PBS (pH 7.... | Cell Chem Biol 24: 293-305 (2017) Article DOI: 10.1016/j.chembiol.2017.01.006 BindingDB Entry DOI: 10.7270/Q2GX49FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 [29-209] (Homo sapiens (Human)) | BDBM223307 (123C3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.22E+3 | -32.3 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Sanford-Burnham-Prebys Medical Discovery Institute | Assay Description For competitive binding assays, 1 mL of 200 mM EphA4-LBD was pre-incubated with the tested compounds at various concentrations in 98 mL of PBS (pH 7.... | Cell Chem Biol 24: 293-305 (2017) Article DOI: 10.1016/j.chembiol.2017.01.006 BindingDB Entry DOI: 10.7270/Q2GX49FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 [29-209] (Homo sapiens (Human)) | BDBM223308 (123C5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.57E+3 | -31.9 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Sanford-Burnham-Prebys Medical Discovery Institute | Assay Description For competitive binding assays, 1 mL of 200 mM EphA4-LBD was pre-incubated with the tested compounds at various concentrations in 98 mL of PBS (pH 7.... | Cell Chem Biol 24: 293-305 (2017) Article DOI: 10.1016/j.chembiol.2017.01.006 BindingDB Entry DOI: 10.7270/Q2GX49FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 [29-209] (Homo sapiens (Human)) | BDBM223302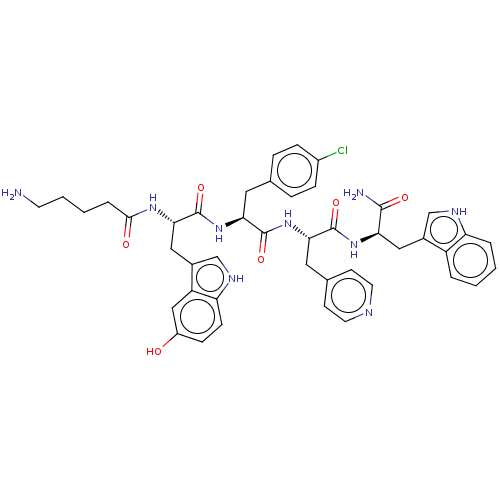 (123B1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.04E+3 | -31.5 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Sanford-Burnham-Prebys Medical Discovery Institute | Assay Description For competitive binding assays, 1 mL of 200 mM EphA4-LBD was pre-incubated with the tested compounds at various concentrations in 98 mL of PBS (pH 7.... | Cell Chem Biol 24: 293-305 (2017) Article DOI: 10.1016/j.chembiol.2017.01.006 BindingDB Entry DOI: 10.7270/Q2GX49FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 [29-209] (Homo sapiens (Human)) | BDBM223309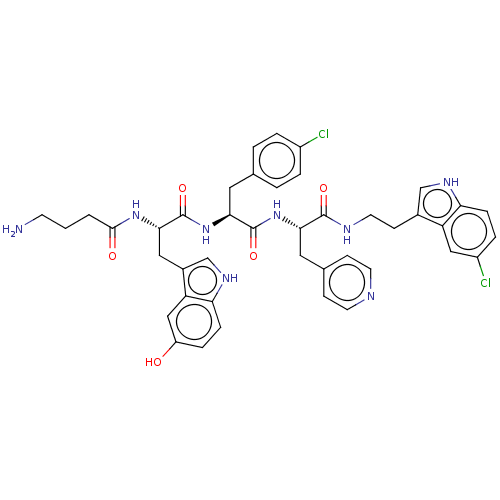 (123C6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.14E+3 | -31.4 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Sanford-Burnham-Prebys Medical Discovery Institute | Assay Description For competitive binding assays, 1 mL of 200 mM EphA4-LBD was pre-incubated with the tested compounds at various concentrations in 98 mL of PBS (pH 7.... | Cell Chem Biol 24: 293-305 (2017) Article DOI: 10.1016/j.chembiol.2017.01.006 BindingDB Entry DOI: 10.7270/Q2GX49FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 [29-209] (Homo sapiens (Human)) | BDBM223306 (123C12) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.67E+3 | -29.2 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Sanford-Burnham-Prebys Medical Discovery Institute | Assay Description For competitive binding assays, 1 mL of 200 mM EphA4-LBD was pre-incubated with the tested compounds at various concentrations in 98 mL of PBS (pH 7.... | Cell Chem Biol 24: 293-305 (2017) Article DOI: 10.1016/j.chembiol.2017.01.006 BindingDB Entry DOI: 10.7270/Q2GX49FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 [29-209] (Homo sapiens (Human)) | BDBM223303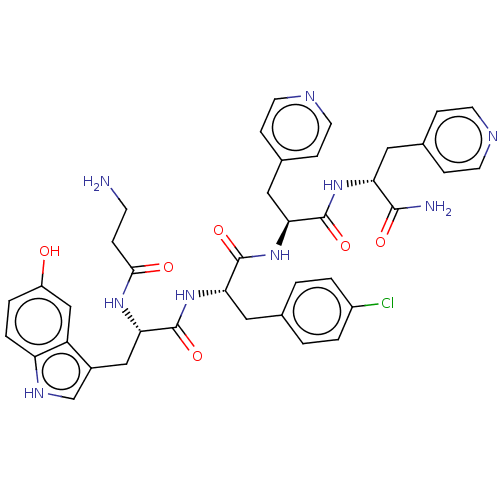 (120G1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40E+4 | -27.7 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Sanford-Burnham-Prebys Medical Discovery Institute | Assay Description For competitive binding assays, 1 mL of 200 mM EphA4-LBD was pre-incubated with the tested compounds at various concentrations in 98 mL of PBS (pH 7.... | Cell Chem Biol 24: 293-305 (2017) Article DOI: 10.1016/j.chembiol.2017.01.006 BindingDB Entry DOI: 10.7270/Q2GX49FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 [29-209] (Homo sapiens (Human)) | BDBM223304 (120G2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+5 | -22.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 25 |
Sanford-Burnham-Prebys Medical Discovery Institute | Assay Description For competitive binding assays, 1 mL of 200 mM EphA4-LBD was pre-incubated with the tested compounds at various concentrations in 98 mL of PBS (pH 7.... | Cell Chem Biol 24: 293-305 (2017) Article DOI: 10.1016/j.chembiol.2017.01.006 BindingDB Entry DOI: 10.7270/Q2GX49FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 [1-949,A922V] (Homo sapiens (Human)) | BDBM82190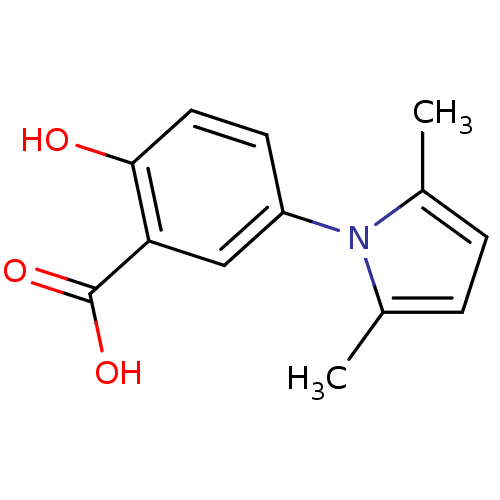 (Salicylic acid derivative, compound 2) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Sanford-Burnham Medical Research Institute | Assay Description Protein A-coasted wells (from Pierce Biotechnology) were used to immobilize Eph receptor Fc fusion proteins (from R&D systems)incubated at 1 ug/ml in... | Chem Biol Drug Des 78: 667-78 (2011) Article DOI: 10.1111/j.1747-0285.2011.01199.x BindingDB Entry DOI: 10.7270/Q2FN14QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 [1-949,A922V] (Homo sapiens (Human)) | BDBM11985 (4-(2,5-dimethyl-1H-pyrrol-1-yl)-2-hydroxybenzoic a...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Sanford-Burnham Medical Research Institute | Assay Description Protein A-coasted wells (from Pierce Biotechnology) were used to immobilize Eph receptor Fc fusion proteins (from R&D systems)incubated at 1 ug/ml in... | Chem Biol Drug Des 78: 667-78 (2011) Article DOI: 10.1111/j.1747-0285.2011.01199.x BindingDB Entry DOI: 10.7270/Q2FN14QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 [1-949,A922V] (Homo sapiens (Human)) | BDBM82189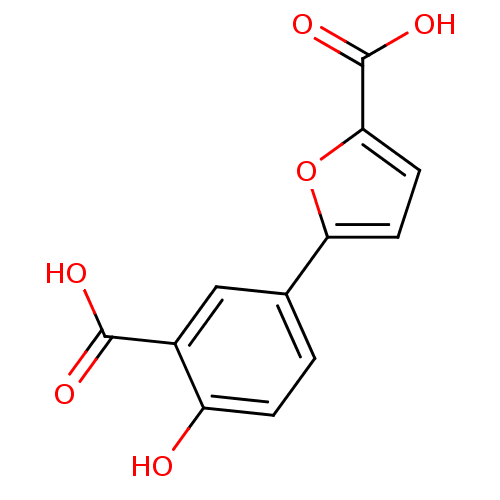 (Salicylic acid derivative, 76B8) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Sanford-Burnham Medical Research Institute | Assay Description Protein A-coasted wells (from Pierce Biotechnology) were used to immobilize Eph receptor Fc fusion proteins (from R&D systems)incubated at 1 ug/ml in... | Chem Biol Drug Des 78: 667-78 (2011) Article DOI: 10.1111/j.1747-0285.2011.01199.x BindingDB Entry DOI: 10.7270/Q2FN14QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 [1-949,A922V] (Homo sapiens (Human)) | BDBM82187 (Salicylic acid derivative, 76D10) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Sanford-Burnham Medical Research Institute | Assay Description Protein A-coasted wells (from Pierce Biotechnology) were used to immobilize Eph receptor Fc fusion proteins (from R&D systems)incubated at 1 ug/ml in... | Chem Biol Drug Des 78: 667-78 (2011) Article DOI: 10.1111/j.1747-0285.2011.01199.x BindingDB Entry DOI: 10.7270/Q2FN14QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 [1-949,A922V] (Homo sapiens (Human)) | BDBM82188 (Salicylic acid derivative, 76A5) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Sanford-Burnham Medical Research Institute | Assay Description Protein A-coasted wells (from Pierce Biotechnology) were used to immobilize Eph receptor Fc fusion proteins (from R&D systems)incubated at 1 ug/ml in... | Chem Biol Drug Des 78: 667-78 (2011) Article DOI: 10.1111/j.1747-0285.2011.01199.x BindingDB Entry DOI: 10.7270/Q2FN14QN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 (Homo sapiens (Human)) | BDBM223311 (123C4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01511 BindingDB Entry DOI: 10.7270/Q22Z19M2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 (Homo sapiens (Human)) | BDBM50463479 (CHEMBL4249925) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of EphA4 (unknown origin) | Bioorg Med Chem Lett 28: 2622-2626 (2018) Article DOI: 10.1016/j.bmcl.2018.06.040 BindingDB Entry DOI: 10.7270/Q2ZC85HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 (Homo sapiens (Human)) | BDBM50463483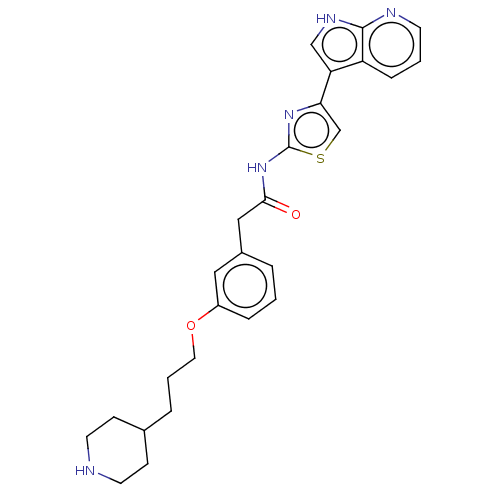 (CHEMBL4245242) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of EphA4 (unknown origin) | Bioorg Med Chem Lett 28: 2622-2626 (2018) Article DOI: 10.1016/j.bmcl.2018.06.040 BindingDB Entry DOI: 10.7270/Q2ZC85HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 (Homo sapiens (Human)) | BDBM4779 (CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q21834VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 (Homo sapiens (Human)) | BDBM13216 (BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PCBioAssay | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q21834VP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ephrin type-A receptor 4 (Homo sapiens (Human)) | BDBM31093 (4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q21834VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 (Homo sapiens (Human)) | BDBM16673 (4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q21834VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 (Homo sapiens (Human)) | BDBM31096 (CHEMBL290084 | Staurosporine | cid_451705) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q21834VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 (Mus musculus) | BDBM44425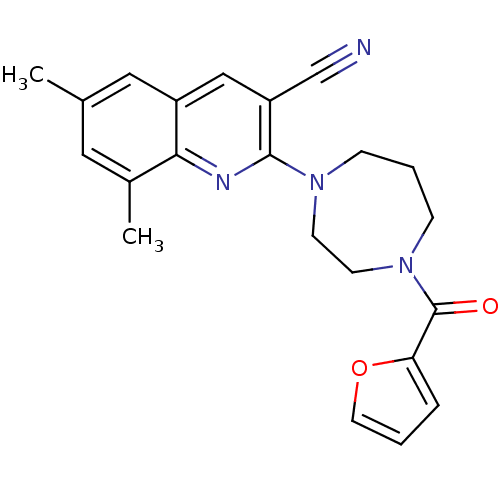 (2-[4-(2-furoyl)-1,4-diazepan-1-yl]-6,8-dimethyl-qu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 1.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2P55KW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 (Mus musculus) | BDBM44426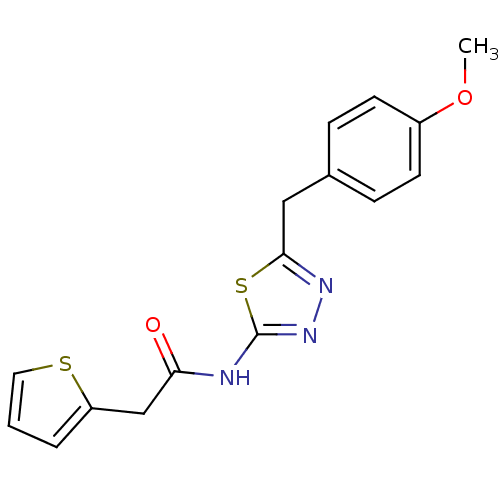 (MLS000027789 | N-(5-p-anisyl-1,3,4-thiadiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2P55KW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 (Mus musculus) | BDBM44427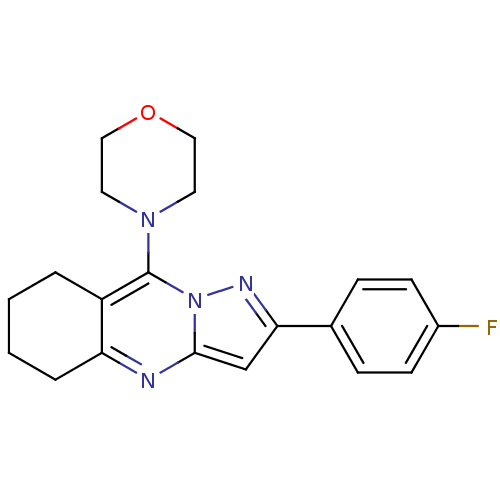 (2-(4-Fluoro-phenyl)-9-morpholin-4-yl-5,6,7,8-tetra...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2P55KW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 (Mus musculus) | BDBM44175 (1-(2-Fluoro-phenyl)-4-[(4-methoxy-phenyl)-(1-thiop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2P55KW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 (Mus musculus) | BDBM38280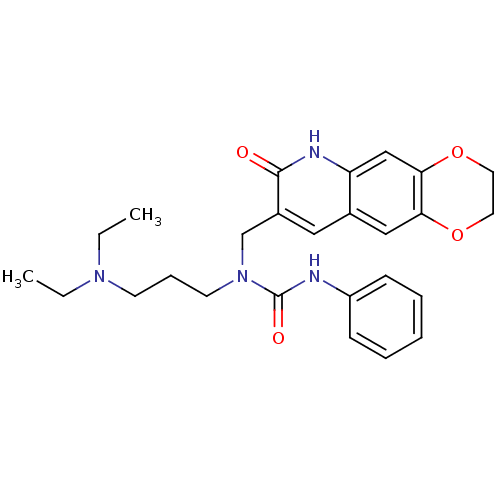 (1-(3-Diethylamino-propyl)-1-(7-oxo-2,3,6,7-tetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2P55KW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 (Mus musculus) | BDBM44428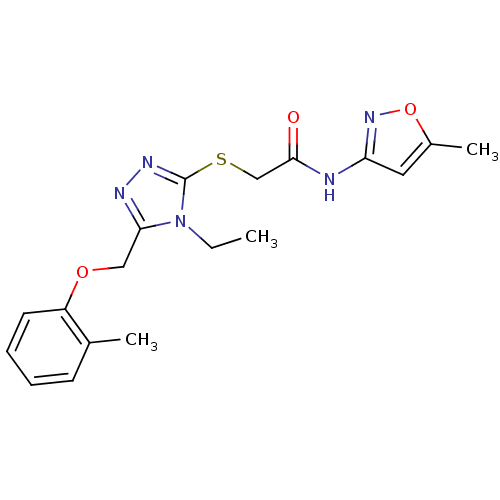 (2-(4-Ethyl-5-o-tolyloxymethyl-4H-[1,2,4]triazol-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2P55KW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 (Mus musculus) | BDBM44429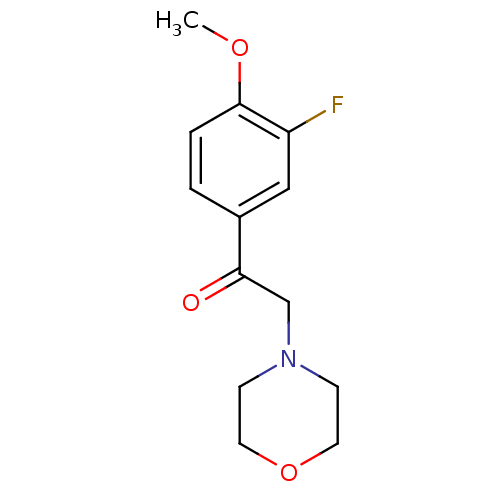 (1-(3-fluoranyl-4-methoxy-phenyl)-2-morpholin-4-yl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2P55KW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 (Mus musculus) | BDBM38802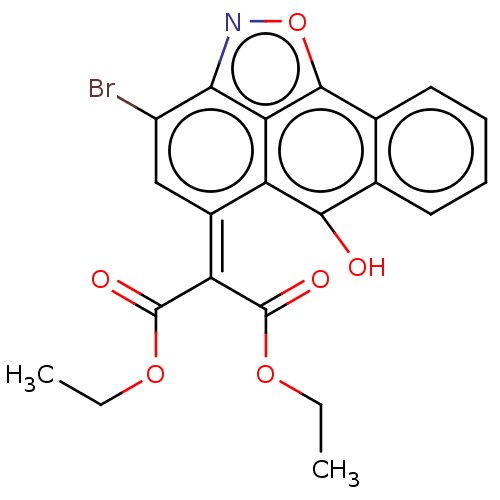 (MLS000038842 | SMR000040243 | cid_659226) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PCBioAssay | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2P55KW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 (Mus musculus) | BDBM44431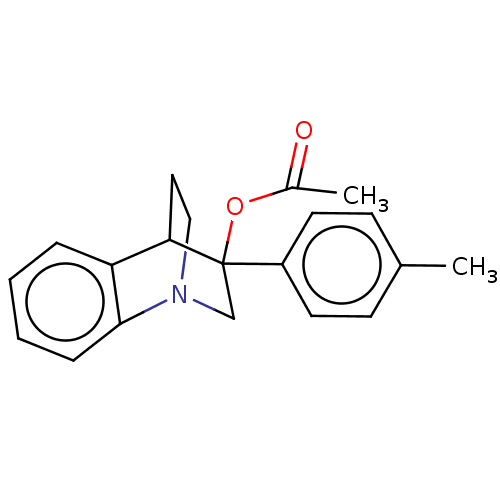 (MLS000038382 | SMR000040281 | cid_6463323) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2P55KW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 (Mus musculus) | BDBM34172 (MLS000038673 | N-(5-ethylsulfanyl-1,3,4-thiadiazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PCBioAssay | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2P55KW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 (Mus musculus) | BDBM44432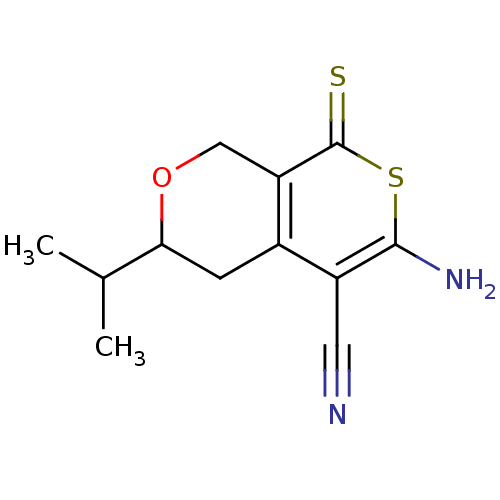 (6-amino-3-isopropyl-8-thioxo-3,4-dihydro-1H-thiopy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 3.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2P55KW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 (Mus musculus) | BDBM31751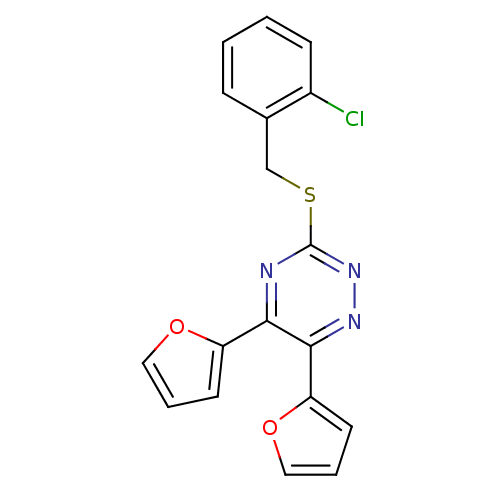 (3-[(2-chlorobenzyl)thio]-5,6-bis(2-furyl)-1,2,4-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 2.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2P55KW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 4 (Mus musculus) | BDBM44434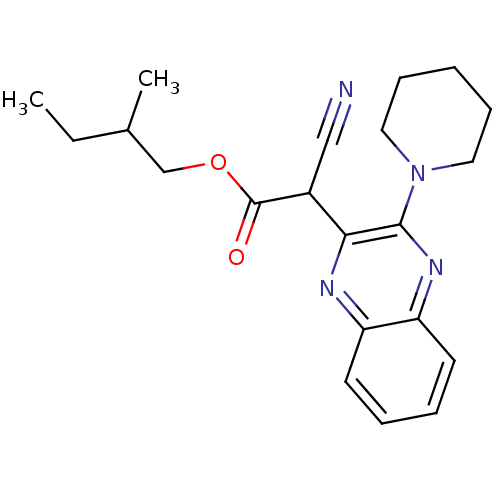 (2-cyano-2-(3-piperidinoquinoxalin-2-yl)acetic acid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q2P55KW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 527 total ) | Next | Last >> |