 Found 2042 hits Enz. Inhib. hit(s) with Target = 'Lactate dehydrogenase A (LDHA)'
Found 2042 hits Enz. Inhib. hit(s) with Target = 'Lactate dehydrogenase A (LDHA)' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM86114
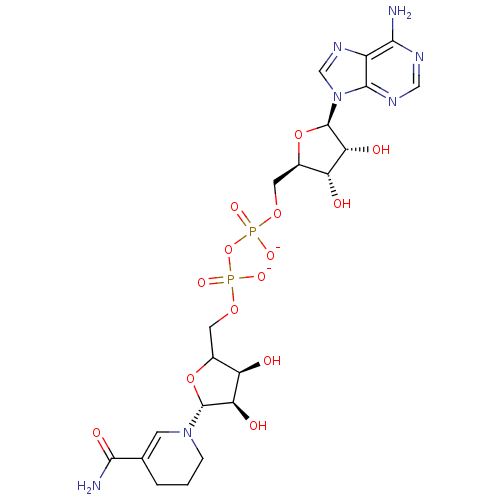
(LDHA Inhibitor, 8)Show SMILES NC(=O)C1=CN(CCC1)[C@@H]1OC(COP([O-])(=O)OP([O-])(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r,t:3| Show InChI InChI=1S/C21H31N7O14P2/c22-17-12-19(25-7-24-17)28(8-26-12)21-16(32)14(30)11(41-21)6-39-44(36,37)42-43(34,35)38-5-10-13(29)15(31)20(40-10)27-3-1-2-9(4-27)18(23)33/h4,7-8,10-11,13-16,20-21,29-32H,1-3,5-6H2,(H2,23,33)(H,34,35)(H,36,37)(H2,22,24,25)/p-2/t10?,11-,13-,14-,15-,16-,20-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.28E+4 | n/a | n/a | n/a | 7.4 | 21 |
AstraZeneca
| Assay Description
A BIAcore 3000 or a BIAcore S51 instrument (GE Healthcare) was used to detect binding interactions using a direct binding assay format. |
J Med Chem 55: 3285-306 (2012)
Article DOI: 10.1021/jm201734r
BindingDB Entry DOI: 10.7270/Q21J9896 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50066974

(7-Benzyl-2,3-dihydroxy-6-methyl-4-propyl-naphthale...)Show SMILES CCCc1c(O)c(O)c(C(O)=O)c2cc(Cc3ccccc3)c(C)cc12 Show InChI InChI=1S/C22H22O4/c1-3-7-16-17-10-13(2)15(11-14-8-5-4-6-9-14)12-18(17)19(22(25)26)21(24)20(16)23/h4-6,8-10,12,23-24H,3,7,11H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 21 |
AstraZeneca
| Assay Description
Enzyme assay using lactate dehydrogenase A (LDHA). |
J Med Chem 55: 3285-306 (2012)
Article DOI: 10.1021/jm201734r
BindingDB Entry DOI: 10.7270/Q21J9896 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50339607
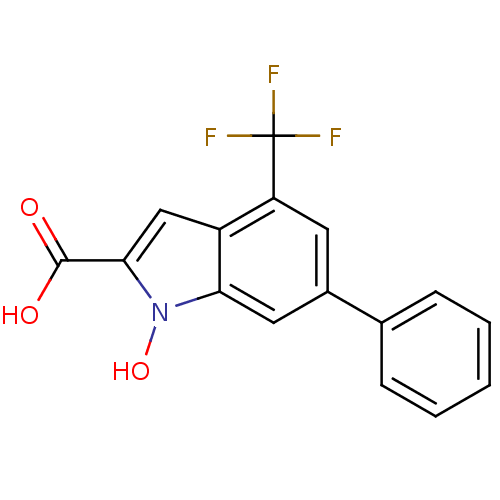
(1-Hydroxy-6-phenyl-4-trifluoromethyl-1H-indole-2-c...)Show InChI InChI=1S/C16H10F3NO3/c17-16(18,19)12-6-10(9-4-2-1-3-5-9)7-13-11(12)8-14(15(21)22)20(13)23/h1-8,23H,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 21 |
AstraZeneca
| Assay Description
Enzyme assay using lactate dehydrogenase A (LDHA). |
J Med Chem 55: 3285-306 (2012)
Article DOI: 10.1021/jm201734r
BindingDB Entry DOI: 10.7270/Q21J9896 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50219487
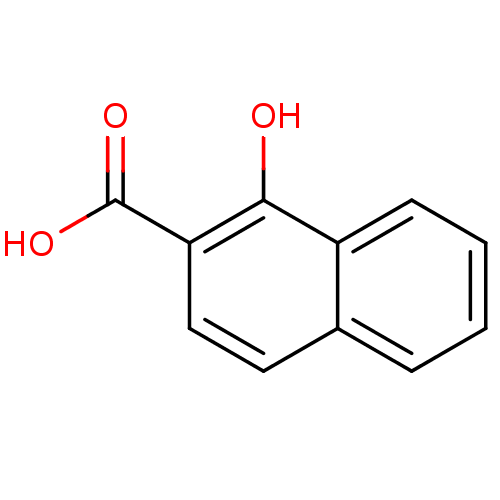
(1-hydroxy-2-naphthoate | 1-hydroxy-2-naphthoic aci...)Show InChI InChI=1S/C11H8O3/c12-10-8-4-2-1-3-7(8)5-6-9(10)11(13)14/h1-6,12H,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 21 |
AstraZeneca
| Assay Description
Enzyme assay using lactate dehydrogenase A (LDHA). |
J Med Chem 55: 3285-306 (2012)
Article DOI: 10.1021/jm201734r
BindingDB Entry DOI: 10.7270/Q21J9896 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50192453
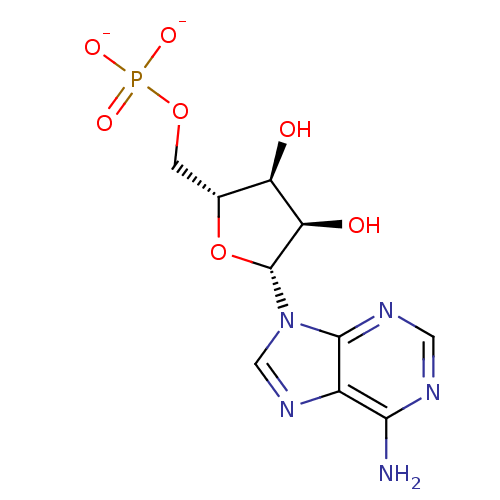
(AMP(2-) | Adenosine-5-monophosphate | LDHA Inhibit...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP([O-])([O-])=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H14N5O7P/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(22-10)1-21-23(18,19)20/h2-4,6-7,10,16-17H,1H2,(H2,11,12,13)(H2,18,19,20)/p-2/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 21 |
AstraZeneca
| Assay Description
Enzyme assay using lactate dehydrogenase A (LDHA). |
J Med Chem 55: 3285-306 (2012)
Article DOI: 10.1021/jm201734r
BindingDB Entry DOI: 10.7270/Q21J9896 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM86113
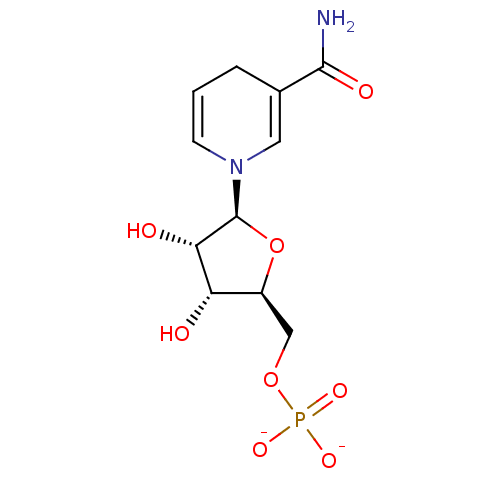
(LDHA Inhibitor, 7)Show SMILES NC(=O)C1=CN(C=CC1)[C@H]1O[C@@H](COP([O-])([O-])=O)[C@H](O)[C@@H]1O |r,c:6,t:3| Show InChI InChI=1S/C11H17N2O8P/c12-10(16)6-2-1-3-13(4-6)11-9(15)8(14)7(21-11)5-20-22(17,18)19/h1,3-4,7-9,11,14-15H,2,5H2,(H2,12,16)(H2,17,18,19)/p-2/t7-,8-,9-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 4.00E+6 | n/a | n/a | n/a | 7.5 | 21 |
AstraZeneca
| Assay Description
NMR spectra were acquired on Bruker Avance 600 MHz spectrometers at 298 K using a 5 mm triple-resonance HCN cryoprobe. Ligand binding was detected u... |
J Med Chem 55: 3285-306 (2012)
Article DOI: 10.1021/jm201734r
BindingDB Entry DOI: 10.7270/Q21J9896 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM86114

(LDHA Inhibitor, 8)Show SMILES NC(=O)C1=CN(CCC1)[C@@H]1OC(COP([O-])(=O)OP([O-])(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r,t:3| Show InChI InChI=1S/C21H31N7O14P2/c22-17-12-19(25-7-24-17)28(8-26-12)21-16(32)14(30)11(41-21)6-39-44(36,37)42-43(34,35)38-5-10-13(29)15(31)20(40-10)27-3-1-2-9(4-27)18(23)33/h4,7-8,10-11,13-16,20-21,29-32H,1-3,5-6H2,(H2,23,33)(H,34,35)(H,36,37)(H2,22,24,25)/p-2/t10?,11-,13-,14-,15-,16-,20-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.32E+5 | n/a | n/a | n/a | n/a | 7.5 | 21 |
AstraZeneca
| Assay Description
Enzyme assay using lactate dehydrogenase A (LDHA). |
J Med Chem 55: 3285-306 (2012)
Article DOI: 10.1021/jm201734r
BindingDB Entry DOI: 10.7270/Q21J9896 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50339607

(1-Hydroxy-6-phenyl-4-trifluoromethyl-1H-indole-2-c...)Show InChI InChI=1S/C16H10F3NO3/c17-16(18,19)12-6-10(9-4-2-1-3-5-9)7-13-11(12)8-14(15(21)22)20(13)23/h1-8,23H,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | 7.5 | 21 |
AstraZeneca
| Assay Description
NMR spectra were acquired on Bruker Avance 600 MHz spectrometers at 298 K using a 5 mm triple-resonance HCN cryoprobe. Ligand binding was detected u... |
J Med Chem 55: 3285-306 (2012)
Article DOI: 10.1021/jm201734r
BindingDB Entry DOI: 10.7270/Q21J9896 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50219487

(1-hydroxy-2-naphthoate | 1-hydroxy-2-naphthoic aci...)Show InChI InChI=1S/C11H8O3/c12-10-8-4-2-1-3-7(8)5-6-9(10)11(13)14/h1-6,12H,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.40E+5 | n/a | n/a | n/a | 7.5 | 21 |
AstraZeneca
| Assay Description
NMR spectra were acquired on Bruker Avance 600 MHz spectrometers at 298 K using a 5 mm triple-resonance HCN cryoprobe. Ligand binding was detected u... |
J Med Chem 55: 3285-306 (2012)
Article DOI: 10.1021/jm201734r
BindingDB Entry DOI: 10.7270/Q21J9896 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50192453

(AMP(2-) | Adenosine-5-monophosphate | LDHA Inhibit...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP([O-])([O-])=O)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H14N5O7P/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(22-10)1-21-23(18,19)20/h2-4,6-7,10,16-17H,1H2,(H2,11,12,13)(H2,18,19,20)/p-2/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.50E+5 | n/a | n/a | n/a | 7.5 | 21 |
AstraZeneca
| Assay Description
NMR spectra were acquired on Bruker Avance 600 MHz spectrometers at 298 K using a 5 mm triple-resonance HCN cryoprobe. Ligand binding was detected u... |
J Med Chem 55: 3285-306 (2012)
Article DOI: 10.1021/jm201734r
BindingDB Entry DOI: 10.7270/Q21J9896 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM23251
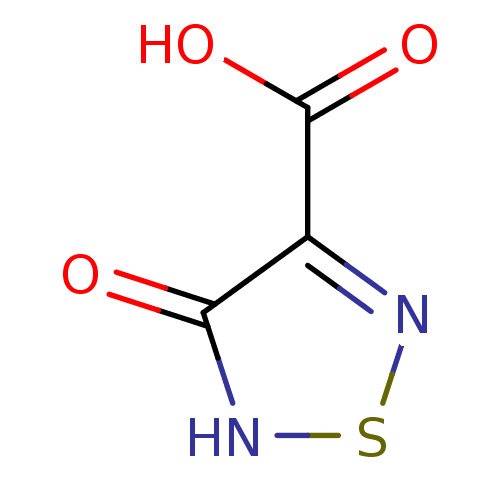
(1,2,5-Thiadiazole, TDA1 | 4-hydroxy-1,2,5-thiadiaz...)Show InChI InChI=1S/C3H2N2O3S/c6-2-1(3(7)8)4-9-5-2/h(H,5,6)(H,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.50E+5 | n/a | n/a | n/a | n/a | 7.5 | 21 |
AstraZeneca
| Assay Description
Enzyme assay using lactate dehydrogenase A (LDHA). |
J Med Chem 55: 3285-306 (2012)
Article DOI: 10.1021/jm201734r
BindingDB Entry DOI: 10.7270/Q21J9896 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM86113

(LDHA Inhibitor, 7)Show SMILES NC(=O)C1=CN(C=CC1)[C@H]1O[C@@H](COP([O-])([O-])=O)[C@H](O)[C@@H]1O |r,c:6,t:3| Show InChI InChI=1S/C11H17N2O8P/c12-10(16)6-2-1-3-13(4-6)11-9(15)8(14)7(21-11)5-20-22(17,18)19/h1,3-4,7-9,11,14-15H,2,5H2,(H2,12,16)(H2,17,18,19)/p-2/t7-,8-,9-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | 7.5 | 21 |
AstraZeneca
| Assay Description
Enzyme assay using lactate dehydrogenase A (LDHA). |
J Med Chem 55: 3285-306 (2012)
Article DOI: 10.1021/jm201734r
BindingDB Entry DOI: 10.7270/Q21J9896 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50103568
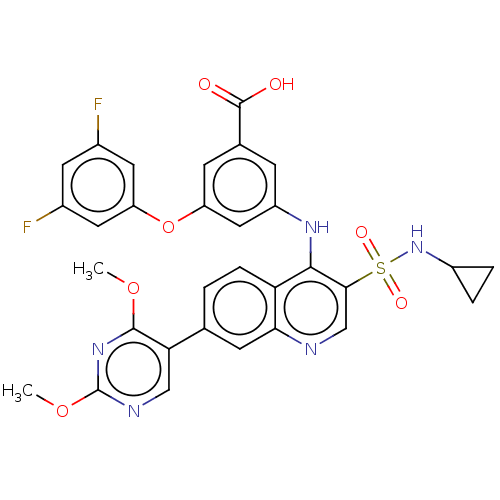
(CHEMBL3335794)Show SMILES COc1ncc(-c2ccc3c(Nc4cc(Oc5cc(F)cc(F)c5)cc(c4)C(O)=O)c(cnc3c2)S(=O)(=O)NC2CC2)c(OC)n1 Show InChI InChI=1S/C31H25F2N5O7S/c1-43-29-25(14-35-31(37-29)44-2)16-3-6-24-26(9-16)34-15-27(46(41,42)38-20-4-5-20)28(24)36-21-7-17(30(39)40)8-22(13-21)45-23-11-18(32)10-19(33)12-23/h3,6-15,20,38H,4-5H2,1-2H3,(H,34,36)(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of LDHA (unknown origin) |
Bioorg Med Chem Lett 24: 4915-25 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.041
BindingDB Entry DOI: 10.7270/Q25Q4XV9 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50144776
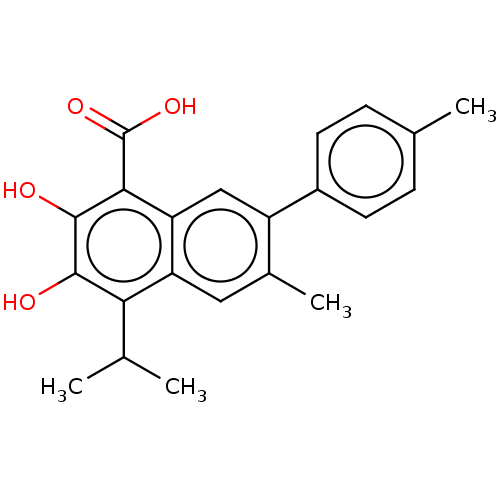
(CHEMBL3763437)Show SMILES CC(C)c1c(O)c(O)c(C(O)=O)c2cc(c(C)cc12)-c1ccc(C)cc1 Show InChI InChI=1S/C22H22O4/c1-11(2)18-16-9-13(4)15(14-7-5-12(3)6-8-14)10-17(16)19(22(25)26)21(24)20(18)23/h5-11,23-24H,1-4H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-CRO
Curated by ChEMBL
| Assay Description
Competitive inhibition of human LDH5 in presence of NADH |
J Med Chem 59: 487-96 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00168
BindingDB Entry DOI: 10.7270/Q2B27X41 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50066974

(7-Benzyl-2,3-dihydroxy-6-methyl-4-propyl-naphthale...)Show SMILES CCCc1c(O)c(O)c(C(O)=O)c2cc(Cc3ccccc3)c(C)cc12 Show InChI InChI=1S/C22H22O4/c1-3-7-16-17-10-13(2)15(11-14-8-5-4-6-9-14)12-18(17)19(22(25)26)21(24)20(16)23/h4-6,8-10,12,23-24H,3,7,11H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-CRO
Curated by ChEMBL
| Assay Description
Competitive inhibition of human LDH5 in presence of NADH |
J Med Chem 59: 487-96 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00168
BindingDB Entry DOI: 10.7270/Q2B27X41 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50594018

(CHEMBL5180187)Show SMILES N.N.OC(=O)c1cc(ccc1O)-c1ccc(CN(Cc2ccc(o2)-c2ccc(O)c(c2)C(O)=O)Cc2ccccc2)o1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50066979
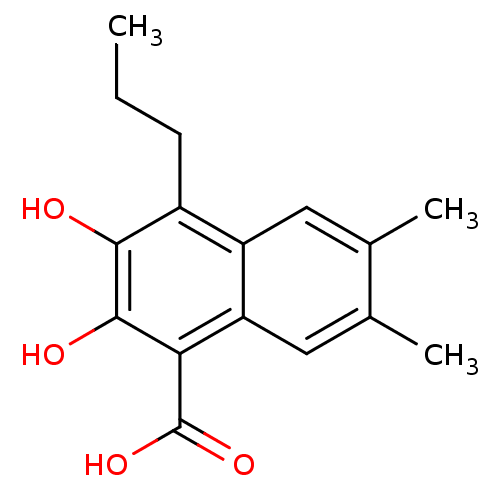
(2,3-Dihydroxy-6,7-dimethyl-4-propyl-naphthalene-1-...)Show InChI InChI=1S/C16H18O4/c1-4-5-10-11-6-8(2)9(3)7-12(11)13(16(19)20)15(18)14(10)17/h6-7,17-18H,4-5H2,1-3H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-CRO
Curated by ChEMBL
| Assay Description
Competitive inhibition of human LDH5 in presence of NADH |
J Med Chem 59: 487-96 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00168
BindingDB Entry DOI: 10.7270/Q2B27X41 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50066979

(2,3-Dihydroxy-6,7-dimethyl-4-propyl-naphthalene-1-...)Show InChI InChI=1S/C16H18O4/c1-4-5-10-11-6-8(2)9(3)7-12(11)13(16(19)20)15(18)14(10)17/h6-7,17-18H,4-5H2,1-3H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
inhibitory activity against P. falciparum lactate dehydrogenase (pLDH) |
J Med Chem 41: 3879-87 (1998)
Article DOI: 10.1021/jm980334n
BindingDB Entry DOI: 10.7270/Q298864G |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50066973

(2,3-Dihydroxy-4-isopropyl-6-methyl-7-(4-trifluorom...)Show SMILES CC(C)c1c(O)c(O)c(C(O)=O)c2cc(Cc3ccc(cc3)C(F)(F)F)c(C)cc12 Show InChI InChI=1S/C23H21F3O4/c1-11(2)18-16-8-12(3)14(9-13-4-6-15(7-5-13)23(24,25)26)10-17(16)19(22(29)30)21(28)20(18)27/h4-8,10-11,27-28H,9H2,1-3H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
inhibitory activity against P. falciparum lactate dehydrogenase (pLDH) |
J Med Chem 41: 3879-87 (1998)
Article DOI: 10.1021/jm980334n
BindingDB Entry DOI: 10.7270/Q298864G |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50144793

(CHEMBL3765804)Show SMILES CC(C)c1c(O)c(O)c(C(O)=O)c2cc(c(C)cc12)-c1cccc(C)c1 Show InChI InChI=1S/C22H22O4/c1-11(2)18-16-9-13(4)15(14-7-5-6-12(3)8-14)10-17(16)19(22(25)26)21(24)20(18)23/h5-11,23-24H,1-4H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-CRO
Curated by ChEMBL
| Assay Description
Competitive inhibition of human LDH5 in presence of NADH |
J Med Chem 59: 487-96 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00168
BindingDB Entry DOI: 10.7270/Q2B27X41 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50066975

(7-Benzyl-2,3-dihydroxy-4-isopropyl-6-methyl-naphth...)Show SMILES CC(C)c1c(O)c(O)c(C(O)=O)c2cc(Cc3ccccc3)c(C)cc12 Show InChI InChI=1S/C22H22O4/c1-12(2)18-16-9-13(3)15(10-14-7-5-4-6-8-14)11-17(16)19(22(25)26)21(24)20(18)23/h4-9,11-12,23-24H,10H2,1-3H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-CRO
Curated by ChEMBL
| Assay Description
Competitive inhibition of human LDH5 in presence of NADH |
J Med Chem 59: 487-96 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00168
BindingDB Entry DOI: 10.7270/Q2B27X41 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50066974

(7-Benzyl-2,3-dihydroxy-6-methyl-4-propyl-naphthale...)Show SMILES CCCc1c(O)c(O)c(C(O)=O)c2cc(Cc3ccccc3)c(C)cc12 Show InChI InChI=1S/C22H22O4/c1-3-7-16-17-10-13(2)15(11-14-8-5-4-6-9-14)12-18(17)19(22(25)26)21(24)20(16)23/h4-6,8-10,12,23-24H,3,7,11H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
inhibitory activity against P. falciparum lactate dehydrogenase (pLDH) |
J Med Chem 41: 3879-87 (1998)
Article DOI: 10.1021/jm980334n
BindingDB Entry DOI: 10.7270/Q298864G |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50066976

(7-Benzyl-2,3-dihydroxy-4,6-dimethyl-naphthalene-1-...)Show InChI InChI=1S/C20H18O4/c1-11-8-15-12(2)18(21)19(22)17(20(23)24)16(15)10-14(11)9-13-6-4-3-5-7-13/h3-8,10,21-22H,9H2,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-CRO
Curated by ChEMBL
| Assay Description
Competitive inhibition of human LDH5 in presence of NADH |
J Med Chem 59: 487-96 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00168
BindingDB Entry DOI: 10.7270/Q2B27X41 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50010449
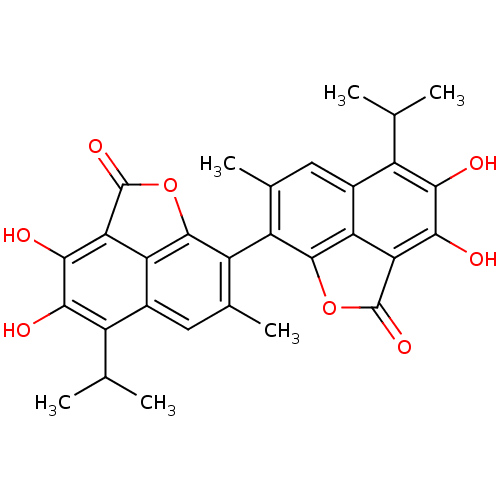
(3,4,3',4'-Tetrahydroxy-5,5'-diisopropyl-7,7'-dimet...)Show SMILES CC(C)c1c(O)c(O)c2C(=O)Oc3c(c(C)cc1c23)-c1c2OC(=O)c3c(O)c(O)c(C(C)C)c(cc1C)c23 |(17.54,-15.98,;16.19,-15.21,;14.86,-15.98,;16.19,-13.65,;17.54,-12.91,;18.85,-13.69,;17.54,-11.35,;18.85,-10.58,;16.19,-10.58,;16.18,-9.03,;17.26,-7.93,;13.55,-9.03,;13.55,-10.58,;12.21,-11.35,;12.21,-12.91,;12.24,-14.01,;13.55,-13.65,;14.86,-12.91,;14.86,-11.35,;9.55,-11.35,;8.21,-10.58,;8.21,-9.03,;5.59,-9.03,;4.5,-7.93,;5.59,-10.58,;4.26,-11.35,;2.92,-10.58,;4.26,-12.91,;2.92,-13.69,;5.59,-13.65,;5.59,-15.21,;6.92,-15.98,;4.26,-15.98,;6.92,-12.91,;8.21,-13.65,;9.55,-12.91,;9.55,-14.01,;6.92,-11.35,)| Show InChI InChI=1S/C30H26O8/c1-9(2)15-13-7-11(5)17(27-19(13)21(29(35)37-27)25(33)23(15)31)18-12(6)8-14-16(10(3)4)24(32)26(34)22-20(14)28(18)38-30(22)36/h7-10,31-34H,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-CRO
Curated by ChEMBL
| Assay Description
Competitive inhibition of human LDH5 in presence of NADH |
J Med Chem 59: 487-96 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00168
BindingDB Entry DOI: 10.7270/Q2B27X41 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50066975

(7-Benzyl-2,3-dihydroxy-4-isopropyl-6-methyl-naphth...)Show SMILES CC(C)c1c(O)c(O)c(C(O)=O)c2cc(Cc3ccccc3)c(C)cc12 Show InChI InChI=1S/C22H22O4/c1-12(2)18-16-9-13(3)15(10-14-7-5-4-6-8-14)11-17(16)19(22(25)26)21(24)20(18)23/h4-9,11-12,23-24H,10H2,1-3H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
inhibitory activity against P. falciparum lactate dehydrogenase (pLDH) |
J Med Chem 41: 3879-87 (1998)
Article DOI: 10.1021/jm980334n
BindingDB Entry DOI: 10.7270/Q298864G |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50594014
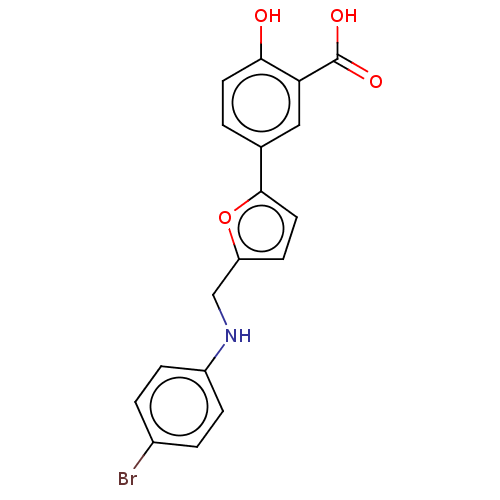
(CHEMBL5182027)Show SMILES N.OC(=O)c1cc(ccc1O)-c1ccc(CNc2ccc(Br)cc2)o1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50066981
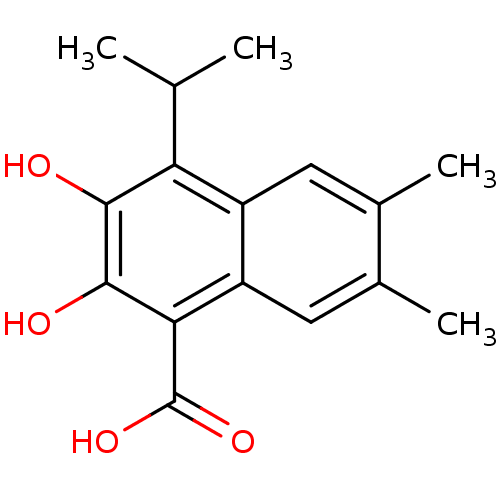
(2,3-Dihydroxy-4-isopropyl-6,7-dimethyl-naphthalene...)Show InChI InChI=1S/C16H18O4/c1-7(2)12-10-5-8(3)9(4)6-11(10)13(16(19)20)15(18)14(12)17/h5-7,17-18H,1-4H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
inhibitory activity against P. falciparum lactate dehydrogenase (pLDH) |
J Med Chem 41: 3879-87 (1998)
Article DOI: 10.1021/jm980334n
BindingDB Entry DOI: 10.7270/Q298864G |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50066977
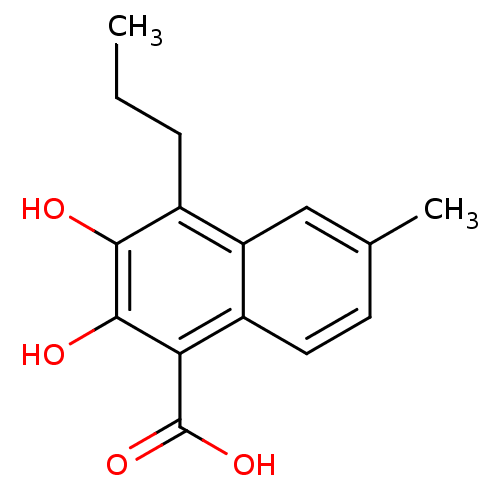
(2,3-Dihydroxy-6-methyl-4-propyl-naphthalene-1-carb...)Show InChI InChI=1S/C15H16O4/c1-3-4-10-11-7-8(2)5-6-9(11)12(15(18)19)14(17)13(10)16/h5-7,16-17H,3-4H2,1-2H3,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-CRO
Curated by ChEMBL
| Assay Description
Competitive inhibition of human LDH5 in presence of NADH |
J Med Chem 59: 487-96 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00168
BindingDB Entry DOI: 10.7270/Q2B27X41 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50144770
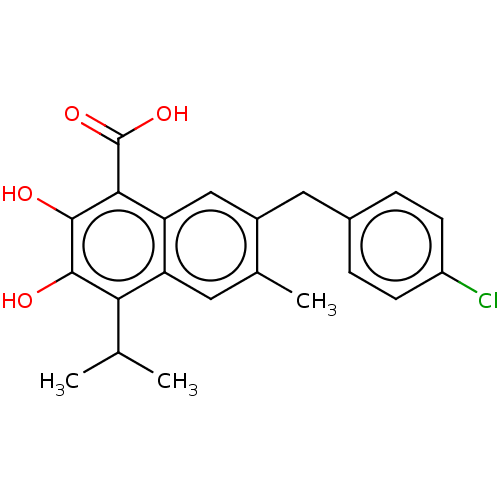
(CHEMBL3765137)Show SMILES CC(C)c1c(O)c(O)c(C(O)=O)c2cc(Cc3ccc(Cl)cc3)c(C)cc12 Show InChI InChI=1S/C22H21ClO4/c1-11(2)18-16-8-12(3)14(9-13-4-6-15(23)7-5-13)10-17(16)19(22(26)27)21(25)20(18)24/h4-8,10-11,24-25H,9H2,1-3H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-CRO
Curated by ChEMBL
| Assay Description
Competitive inhibition of human LDH5 in presence of NADH |
J Med Chem 59: 487-96 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00168
BindingDB Entry DOI: 10.7270/Q2B27X41 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50139139
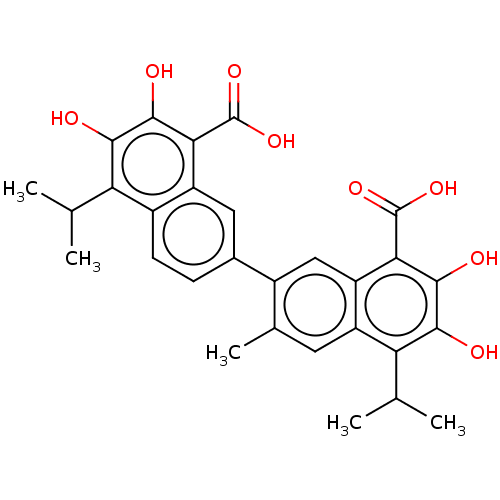
(CHEMBL3765374)Show SMILES CC(C)c1c(O)c(O)c(C(O)=O)c2cc(ccc12)-c1cc2c(C(O)=O)c(O)c(O)c(C(C)C)c2cc1C Show InChI InChI=1S/C29H28O8/c1-11(2)20-15-7-6-14(9-18(15)22(28(34)35)26(32)24(20)30)16-10-19-17(8-13(16)5)21(12(3)4)25(31)27(33)23(19)29(36)37/h6-12,30-33H,1-5H3,(H,34,35)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-CRO
Curated by ChEMBL
| Assay Description
Competitive inhibition of human LDH5 in presence of NADH |
J Med Chem 59: 487-96 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00168
BindingDB Entry DOI: 10.7270/Q2B27X41 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50463040

(CHEMBL4247590)Show InChI InChI=1S/C12H8O5/c13-6-8-2-4-11(17-8)7-1-3-10(14)9(5-7)12(15)16/h1-6,14H,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
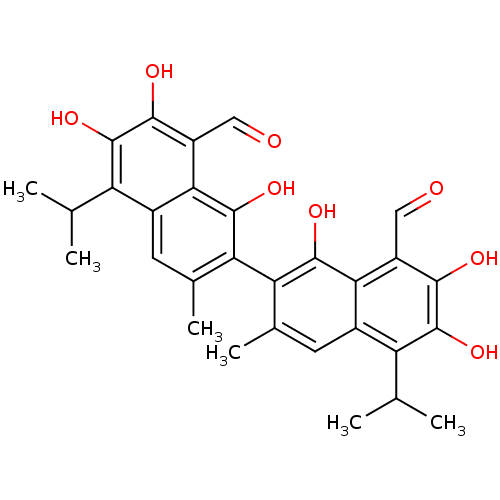
(Homo sapiens (Human)) | BDBM23223
(7-[8-formyl-1,6,7-trihydroxy-3-methyl-5-(propan-2-...)Show SMILES CC(C)c1c(O)c(O)c(C=O)c2c(O)c(c(C)cc12)-c1c(C)cc2c(C(C)C)c(O)c(O)c(C=O)c2c1O |(-4.44,-1.63,;-5.78,-.86,;-7.11,-1.63,;-5.78,.68,;-7.11,1.45,;-8.44,.68,;-7.11,2.99,;-8.44,3.76,;-5.78,3.76,;-5.78,5.3,;-4.44,6.07,;-4.44,2.99,;-3.11,3.76,;-3.11,5.3,;-1.77,2.99,;-1.77,1.45,;-.44,.68,;-3.11,.68,;-4.44,1.45,;-.44,3.76,;-.44,5.3,;-1.77,6.07,;.89,6.07,;2.23,5.3,;3.56,6.07,;3.56,7.61,;4.89,8.38,;2.23,8.38,;4.89,5.3,;6.23,6.07,;4.89,3.76,;6.23,2.99,;3.56,2.99,;3.56,1.45,;4.89,.68,;2.23,3.76,;.89,2.99,;.89,1.45,)| Show InChI InChI=1S/C30H30O8/c1-11(2)19-15-7-13(5)21(27(35)23(15)17(9-31)25(33)29(19)37)22-14(6)8-16-20(12(3)4)30(38)26(34)18(10-32)24(16)28(22)36/h7-12,33-38H,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114605
BindingDB Entry DOI: 10.7270/Q2VM4H8W |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM23223

(7-[8-formyl-1,6,7-trihydroxy-3-methyl-5-(propan-2-...)Show SMILES CC(C)c1c(O)c(O)c(C=O)c2c(O)c(c(C)cc12)-c1c(C)cc2c(C(C)C)c(O)c(O)c(C=O)c2c1O |(-4.44,-1.63,;-5.78,-.86,;-7.11,-1.63,;-5.78,.68,;-7.11,1.45,;-8.44,.68,;-7.11,2.99,;-8.44,3.76,;-5.78,3.76,;-5.78,5.3,;-4.44,6.07,;-4.44,2.99,;-3.11,3.76,;-3.11,5.3,;-1.77,2.99,;-1.77,1.45,;-.44,.68,;-3.11,.68,;-4.44,1.45,;-.44,3.76,;-.44,5.3,;-1.77,6.07,;.89,6.07,;2.23,5.3,;3.56,6.07,;3.56,7.61,;4.89,8.38,;2.23,8.38,;4.89,5.3,;6.23,6.07,;4.89,3.76,;6.23,2.99,;3.56,2.99,;3.56,1.45,;4.89,.68,;2.23,3.76,;.89,2.99,;.89,1.45,)| Show InChI InChI=1S/C30H30O8/c1-11(2)19-15-7-13(5)21(27(35)23(15)17(9-31)25(33)29(19)37)22-14(6)8-16-20(12(3)4)30(38)26(34)18(10-32)24(16)28(22)36/h7-12,33-38H,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-CRO
Curated by ChEMBL
| Assay Description
Competitive inhibition of human LDH5 in presence of NADH |
J Med Chem 59: 487-96 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00168
BindingDB Entry DOI: 10.7270/Q2B27X41 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50594017
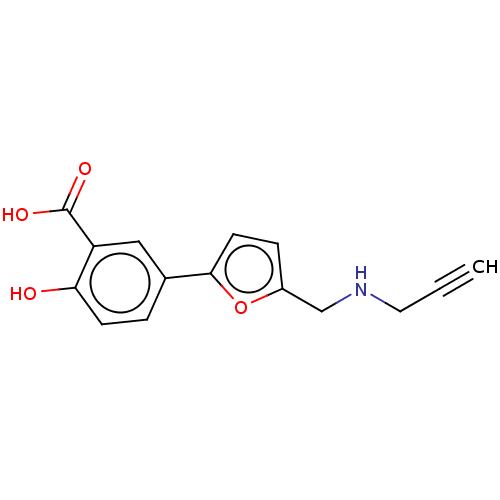
(CHEMBL5190607) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50010438

(Acetic acid 1'-acetoxy-8,8'-dicyano-6,7,6',7'-tetr...)Show SMILES CC(C)c1c(O)c(O)c(C#N)c2c(OC(C)=O)c(c(C)cc12)-c1c(C)cc2c(C(C)C)c(O)c(O)c(C#N)c2c1OC(C)=O |(.71,-9.15,;2.04,-8.36,;3.37,-9.15,;2.04,-6.82,;.71,-6.07,;-.65,-6.84,;.71,-4.51,;-.65,-3.72,;2.04,-3.74,;2.04,-2.2,;2.04,-.66,;3.37,-4.51,;4.67,-3.74,;4.67,-2.2,;6,-1.42,;7.33,-2.18,;5.98,.12,;6,-4.51,;6,-6.07,;6,-7.17,;4.67,-6.82,;3.37,-6.07,;8.66,-4.51,;8.66,-6.07,;8.68,-7.17,;9.99,-6.82,;11.29,-6.07,;12.61,-6.82,;12.61,-8.36,;13.96,-9.15,;11.29,-9.15,;13.96,-6.07,;15.31,-6.84,;13.96,-4.51,;15.31,-3.72,;12.61,-3.74,;12.61,-2.2,;12.61,-.66,;11.29,-4.51,;9.99,-3.74,;9.99,-2.2,;8.89,-1.1,;7.4,-1.49,;9.29,.39,)| Show InChI InChI=1S/C34H32N2O8/c1-13(2)23-19-9-15(5)25(33(43-17(7)37)27(19)21(11-35)29(39)31(23)41)26-16(6)10-20-24(14(3)4)32(42)30(40)22(12-36)28(20)34(26)44-18(8)38/h9-10,13-14,39-42H,1-8H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-CRO
Curated by ChEMBL
| Assay Description
Competitive inhibition of human LDH5 in presence of NADH |
J Med Chem 59: 487-96 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00168
BindingDB Entry DOI: 10.7270/Q2B27X41 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50031638

(2,3-Dihydroxy-4-isopropyl-6-methyl-naphthalene-1-c...)Show InChI InChI=1S/C15H16O4/c1-7(2)11-10-6-8(3)4-5-9(10)12(15(18)19)14(17)13(11)16/h4-7,16-17H,1-3H3,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
inhibitory activity against P. falciparum lactate dehydrogenase (pLDH) |
J Med Chem 41: 3879-87 (1998)
Article DOI: 10.1021/jm980334n
BindingDB Entry DOI: 10.7270/Q298864G |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50066981

(2,3-Dihydroxy-4-isopropyl-6,7-dimethyl-naphthalene...)Show InChI InChI=1S/C16H18O4/c1-7(2)12-10-5-8(3)9(4)6-11(10)13(16(19)20)15(18)14(12)17/h5-7,17-18H,1-4H3,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-CRO
Curated by ChEMBL
| Assay Description
Competitive inhibition of human LDH5 in presence of NADH |
J Med Chem 59: 487-96 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00168
BindingDB Entry DOI: 10.7270/Q2B27X41 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50010448
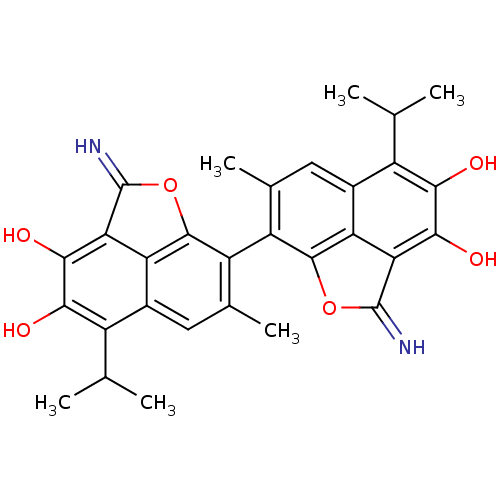
(2,2'-Diimino-5,5'-diisopropyl-7,7'-dimethyl-2H,2'H...)Show SMILES CC(C)c1c(O)c(O)c2C(=N)Oc3c(c(C)cc1c23)-c1c2OC(=N)c3c(O)c(O)c(C(C)C)c(cc1C)c23 |(17.54,-15.98,;16.19,-15.21,;14.86,-15.98,;16.19,-13.65,;17.54,-12.91,;18.85,-13.69,;17.54,-11.35,;18.85,-10.58,;16.19,-10.58,;16.18,-9.03,;17.26,-7.93,;13.55,-9.03,;13.55,-10.58,;12.21,-11.35,;12.21,-12.91,;12.24,-14.01,;13.55,-13.65,;14.86,-12.91,;14.86,-11.35,;9.55,-11.35,;8.21,-10.58,;8.21,-9.03,;5.59,-9.03,;4.5,-7.93,;5.59,-10.58,;4.26,-11.35,;2.92,-10.58,;4.26,-12.91,;2.92,-13.69,;5.59,-13.65,;5.59,-15.21,;6.92,-15.98,;4.26,-15.98,;6.92,-12.91,;8.21,-13.65,;9.55,-12.91,;9.55,-14.01,;6.92,-11.35,)| Show InChI InChI=1S/C30H28N2O6/c1-9(2)15-13-7-11(5)17(27-19(13)21(29(31)37-27)25(35)23(15)33)18-12(6)8-14-16(10(3)4)24(34)26(36)22-20(14)28(18)38-30(22)32/h7-10,31-36H,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-CRO
Curated by ChEMBL
| Assay Description
Competitive inhibition of human LDH5 in presence of NADH |
J Med Chem 59: 487-96 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00168
BindingDB Entry DOI: 10.7270/Q2B27X41 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50144743
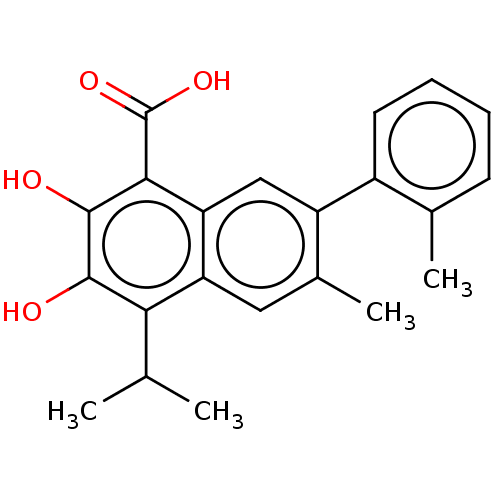
(CHEMBL3764196)Show SMILES CC(C)c1c(O)c(O)c(C(O)=O)c2cc(c(C)cc12)-c1ccccc1C Show InChI InChI=1S/C22H22O4/c1-11(2)18-16-9-13(4)15(14-8-6-5-7-12(14)3)10-17(16)19(22(25)26)21(24)20(18)23/h5-11,23-24H,1-4H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-CRO
Curated by ChEMBL
| Assay Description
Competitive inhibition of human LDH5 in presence of NADH |
J Med Chem 59: 487-96 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00168
BindingDB Entry DOI: 10.7270/Q2B27X41 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50031638

(2,3-Dihydroxy-4-isopropyl-6-methyl-naphthalene-1-c...)Show InChI InChI=1S/C15H16O4/c1-7(2)11-10-6-8(3)4-5-9(10)12(15(18)19)14(17)13(11)16/h4-7,16-17H,1-3H3,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-CRO
Curated by ChEMBL
| Assay Description
Competitive inhibition of human LDH5 in presence of NADH |
J Med Chem 59: 487-96 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00168
BindingDB Entry DOI: 10.7270/Q2B27X41 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50066980
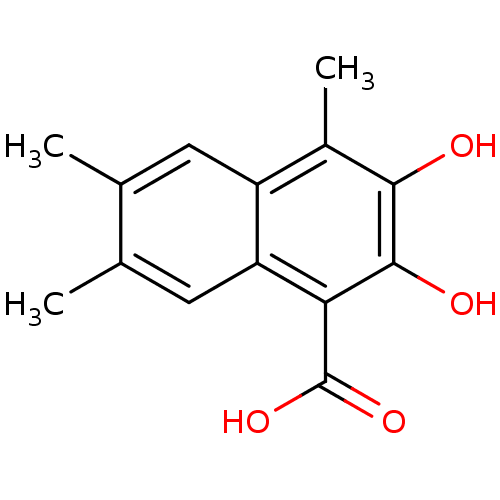
(2,3-Dihydroxy-4,6,7-trimethyl-naphthalene-1-carbox...)Show InChI InChI=1S/C14H14O4/c1-6-4-9-8(3)12(15)13(16)11(14(17)18)10(9)5-7(6)2/h4-5,15-16H,1-3H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-CRO
Curated by ChEMBL
| Assay Description
Competitive inhibition of human LDH5 in presence of NADH |
J Med Chem 59: 487-96 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00168
BindingDB Entry DOI: 10.7270/Q2B27X41 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50339607

(1-Hydroxy-6-phenyl-4-trifluoromethyl-1H-indole-2-c...)Show InChI InChI=1S/C16H10F3NO3/c17-16(18,19)12-6-10(9-4-2-1-3-5-9)7-13-11(12)8-14(15(21)22)20(13)23/h1-8,23H,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` di Pisa
Curated by ChEMBL
| Assay Description
Competitive inhibition of human LDH-A using pyruvate as substrate after 5 mins by calorimetric assay relative to control |
J Med Chem 54: 1599-612 (2011)
Article DOI: 10.1021/jm101007q
BindingDB Entry DOI: 10.7270/Q2J38SVC |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50103570
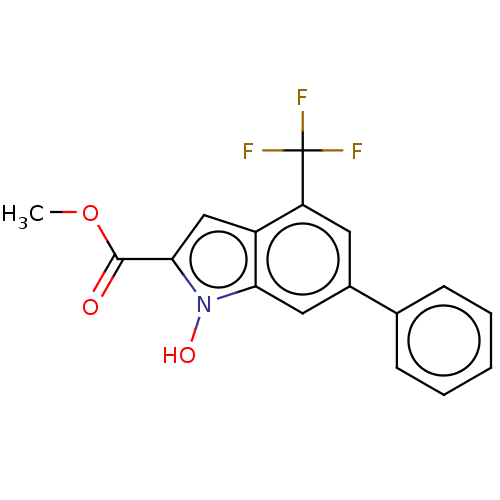
(CHEMBL3335796)Show InChI InChI=1S/C17H12F3NO3/c1-24-16(22)15-9-12-13(17(18,19)20)7-11(8-14(12)21(15)23)10-5-3-2-4-6-10/h2-9,23H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of LDHA (unknown origin) |
Bioorg Med Chem Lett 24: 4915-25 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.041
BindingDB Entry DOI: 10.7270/Q25Q4XV9 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50144747

(CHEMBL3763517)Show InChI InChI=1S/C13H8O7/c14-6-1-4-2-8(16)13(19)20-12(4)9-5(6)3-7(15)10(17)11(9)18/h2-3,15-18H,1H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-CRO
Curated by ChEMBL
| Assay Description
Competitive inhibition of human LDH5 meaured for 3 mins in presence of pyruvate by fluorescence analysis |
J Med Chem 59: 487-96 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00168
BindingDB Entry DOI: 10.7270/Q2B27X41 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50592886

(CHEMBL5199516) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114605
BindingDB Entry DOI: 10.7270/Q2VM4H8W |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50066977

(2,3-Dihydroxy-6-methyl-4-propyl-naphthalene-1-carb...)Show InChI InChI=1S/C15H16O4/c1-3-4-10-11-7-8(2)5-6-9(11)12(15(18)19)14(17)13(10)16/h5-7,16-17H,3-4H2,1-2H3,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
inhibitory activity against P. falciparum lactate dehydrogenase (pLDH) |
J Med Chem 41: 3879-87 (1998)
Article DOI: 10.1021/jm980334n
BindingDB Entry DOI: 10.7270/Q298864G |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50594017

(CHEMBL5190607) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114396
BindingDB Entry DOI: 10.7270/Q2W38196 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50532210

(CHEBI:67078 | DELTA9-TETRAHYDROCANNABINOLIC ACID A)Show SMILES [H][C@@]12CCC(C)=C[C@@]1([H])c1c(O)c(C(O)=O)c(CCCCC)cc1OC2(C)C |r,c:5| Show InChI InChI=1S/C22H30O4/c1-5-6-7-8-14-12-17-19(20(23)18(14)21(24)25)15-11-13(2)9-10-16(15)22(3,4)26-17/h11-12,15-16,23H,5-10H2,1-4H3,(H,24,25)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Non-competitive inhibition of human LDHA assessed as reduction in lactate production using pyruvate as substrate in presence of NADH by Lineweaver-Bu... |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01281
BindingDB Entry DOI: 10.7270/Q29G5RNR |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50066976

(7-Benzyl-2,3-dihydroxy-4,6-dimethyl-naphthalene-1-...)Show InChI InChI=1S/C20H18O4/c1-11-8-15-12(2)18(21)19(22)17(20(23)24)16(15)10-14(11)9-13-6-4-3-5-7-13/h3-8,10,21-22H,9H2,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine
Curated by ChEMBL
| Assay Description
inhibitory activity against P. falciparum lactate dehydrogenase (pLDH) |
J Med Chem 41: 3879-87 (1998)
Article DOI: 10.1021/jm980334n
BindingDB Entry DOI: 10.7270/Q298864G |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50066974

(7-Benzyl-2,3-dihydroxy-6-methyl-4-propyl-naphthale...)Show SMILES CCCc1c(O)c(O)c(C(O)=O)c2cc(Cc3ccccc3)c(C)cc12 Show InChI InChI=1S/C22H22O4/c1-3-7-16-17-10-13(2)15(11-14-8-5-4-6-9-14)12-18(17)19(22(25)26)21(24)20(16)23/h4-6,8-10,12,23-24H,3,7,11H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chongqing University
Curated by ChEMBL
| Assay Description
Inhibition of human LDHA using pyruvate as substrate assessed as disappearance of NADH |
Bioorg Med Chem Lett 26: 72-5 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.025
BindingDB Entry DOI: 10.7270/Q2X63R09 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data