

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM497267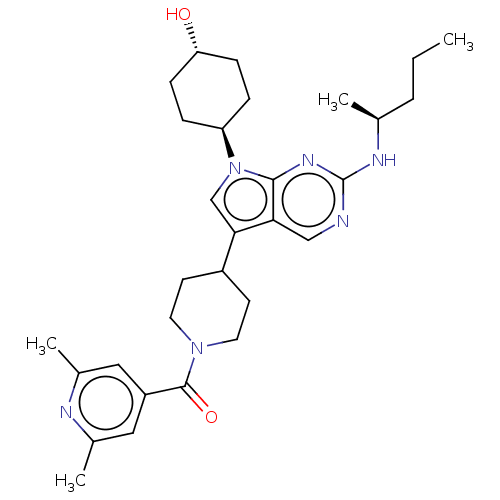 ((2,6-dimethylpyridin-4- yl)(4-(7-((1R,4S)-4- hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ATP competitive inhibition of MERTK (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113534 BindingDB Entry DOI: 10.7270/Q2M90DGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50055499 (CHEMBL3326002) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer (unknown origin) by Off-chip Mobility Shift Assay | J Med Chem 57: 7031-41 (2014) Article DOI: 10.1021/jm500749d BindingDB Entry DOI: 10.7270/Q2K075XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50469353 (CHEMBL4283353) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNC Eshelman School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of MERTK (unknown origin) using 5'-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by MCE assay | J Med Chem 61: 10242-10254 (2018) Article DOI: 10.1021/acs.jmedchem.8b01229 BindingDB Entry DOI: 10.7270/Q2K076ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50384584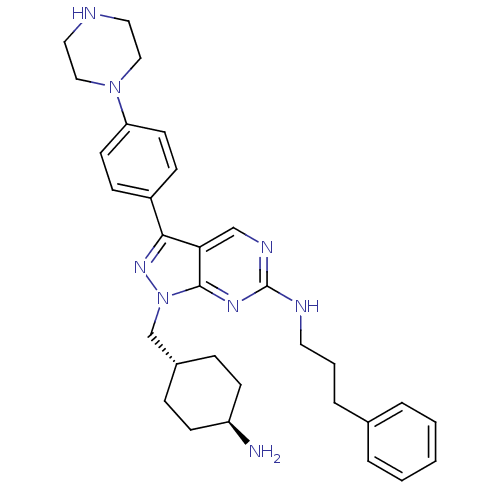 (CHEMBL2036807 | US9744172, Compound UNC607A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Mer using EFPIYDFLPAKKK-CONH2 as substrate by Michaelis-Menton equation | ACS Med Chem Lett 3: 129-134 (2012) Article DOI: 10.1021/ml200239k BindingDB Entry DOI: 10.7270/Q2F76DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50384583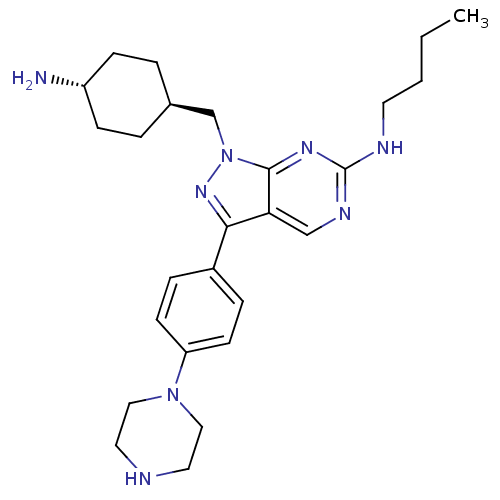 (CHEMBL2036806) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Mer using EFPIYDFLPAKKK-CONH2 as substrate by Michaelis-Menton equation | ACS Med Chem Lett 3: 129-134 (2012) Article DOI: 10.1021/ml200239k BindingDB Entry DOI: 10.7270/Q2F76DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50384582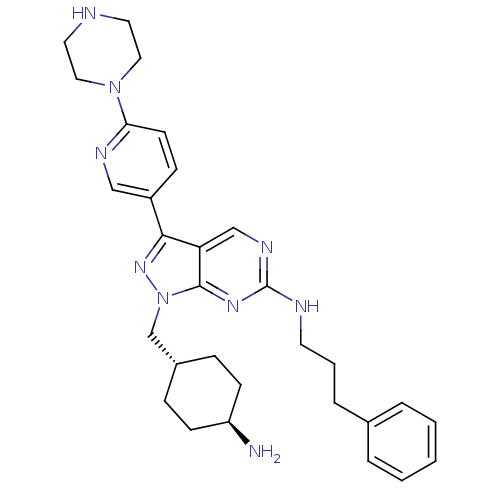 (CHEMBL2036805) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Mer using EFPIYDFLPAKKK-CONH2 as substrate by Michaelis-Menton equation | ACS Med Chem Lett 3: 129-134 (2012) Article DOI: 10.1021/ml200239k BindingDB Entry DOI: 10.7270/Q2F76DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50384585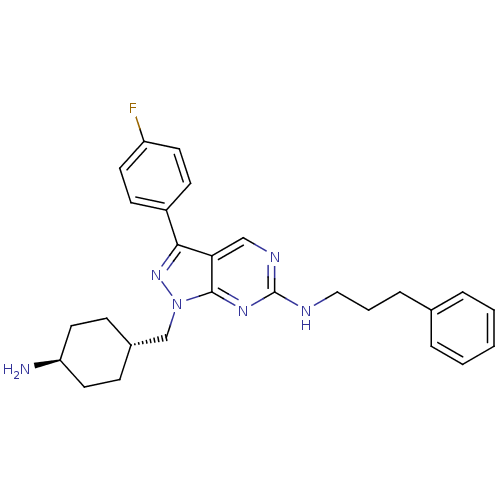 (CHEMBL2036809) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Mer using EFPIYDFLPAKKK-CONH2 as substrate by Michaelis-Menton equation | ACS Med Chem Lett 3: 129-134 (2012) Article DOI: 10.1021/ml200239k BindingDB Entry DOI: 10.7270/Q2F76DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50384581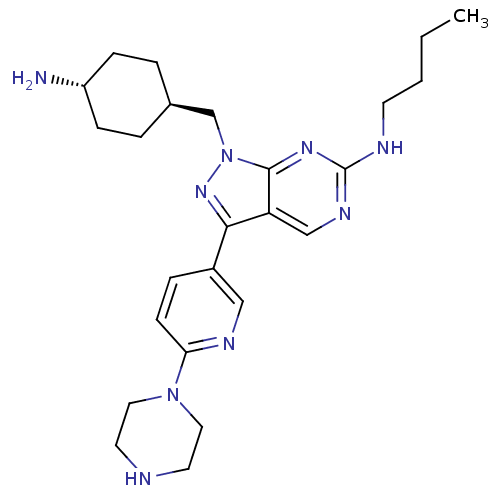 (CHEMBL2036804) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Mer using EFPIYDFLPAKKK-CONH2 as substrate by Michaelis-Menton equation | ACS Med Chem Lett 3: 129-134 (2012) Article DOI: 10.1021/ml200239k BindingDB Entry DOI: 10.7270/Q2F76DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50384576 (CHEMBL2036808) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Mer using EFPIYDFLPAKKK-CONH2 as substrate by Michaelis-Menton equation | ACS Med Chem Lett 3: 129-134 (2012) Article DOI: 10.1021/ml200239k BindingDB Entry DOI: 10.7270/Q2F76DMC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM350085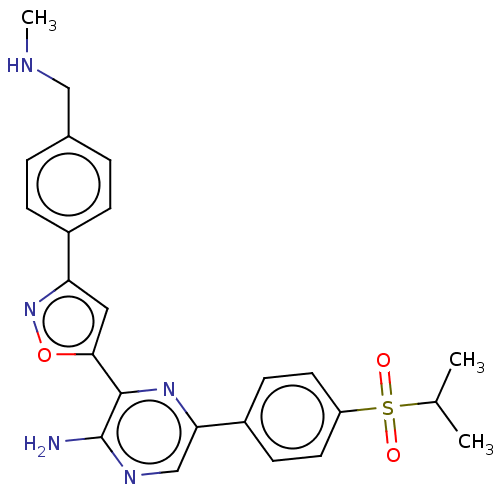 (3-[3-[4-[dideuterio(methylamino)methyl]phenyl]isox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd. Curated by ChEMBL | Assay Description ATP competitive inhibition of human Mer | J Med Chem 62: 5547-5561 (2019) Article DOI: 10.1021/acs.jmedchem.9b00426 BindingDB Entry DOI: 10.7270/Q2BZ69C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50463479 (CHEMBL4249925) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of MER (unknown origin) | Bioorg Med Chem Lett 28: 2622-2626 (2018) Article DOI: 10.1016/j.bmcl.2018.06.040 BindingDB Entry DOI: 10.7270/Q2ZC85HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50463483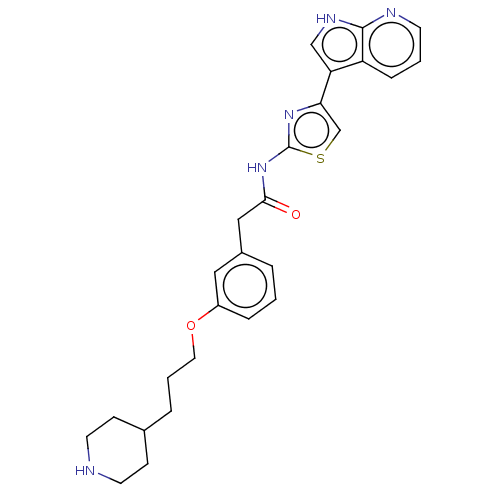 (CHEMBL4245242) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of MER (unknown origin) | Bioorg Med Chem Lett 28: 2622-2626 (2018) Article DOI: 10.1016/j.bmcl.2018.06.040 BindingDB Entry DOI: 10.7270/Q2ZC85HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50431278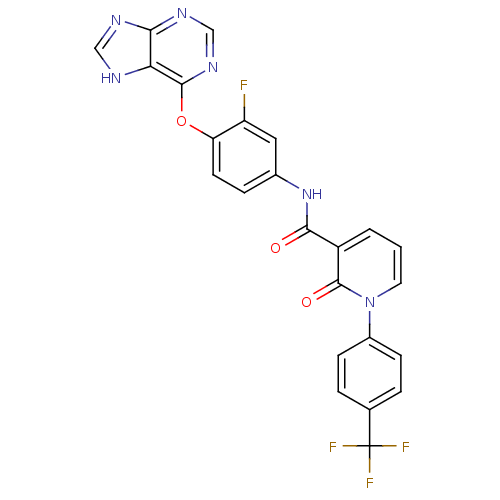 (CHEMBL2348264) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a |
Centre Universitaire Curated by ChEMBL | Assay Description Binding affinity to MER (unknown origin) after 1 hr | Eur J Med Chem 61: 2-25 (2013) Article DOI: 10.1016/j.ejmech.2012.06.005 BindingDB Entry DOI: 10.7270/Q2V40WJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50431279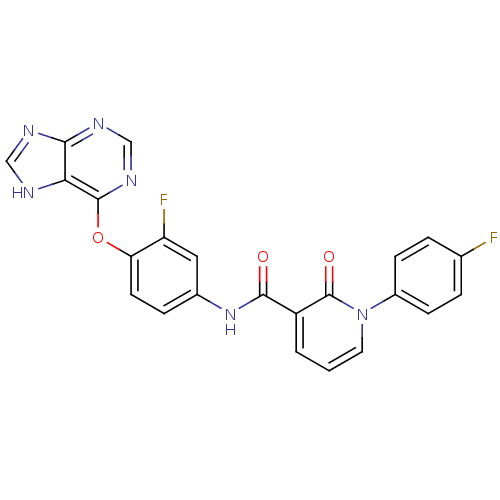 (CHEMBL2346680) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a |
Centre Universitaire Curated by ChEMBL | Assay Description Binding affinity to MER (unknown origin) after 1 hr | Eur J Med Chem 61: 2-25 (2013) Article DOI: 10.1016/j.ejmech.2012.06.005 BindingDB Entry DOI: 10.7270/Q2V40WJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50431280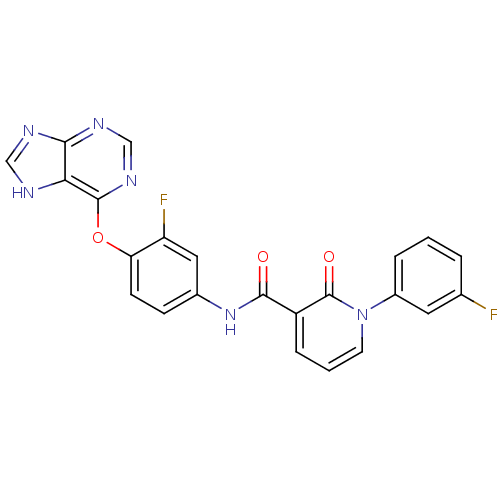 (CHEMBL2348263) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a |
Centre Universitaire Curated by ChEMBL | Assay Description Binding affinity to MER (unknown origin) after 1 hr | Eur J Med Chem 61: 2-25 (2013) Article DOI: 10.1016/j.ejmech.2012.06.005 BindingDB Entry DOI: 10.7270/Q2V40WJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50113707 (2,6,9-Trisubstitute purine, 2 | 2-(2-hydroxyethyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Universitaire Curated by ChEMBL | Assay Description Inhibition of MER (588 to 855) (unknown origin) by fluorescence assay | Eur J Med Chem 61: 2-25 (2013) Article DOI: 10.1016/j.ejmech.2012.06.005 BindingDB Entry DOI: 10.7270/Q2V40WJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50384576 (CHEMBL2036808) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Universitaire Curated by ChEMBL | Assay Description Inhibition of MER (unknown origin) | Eur J Med Chem 61: 2-25 (2013) Article DOI: 10.1016/j.ejmech.2012.06.005 BindingDB Entry DOI: 10.7270/Q2V40WJP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM21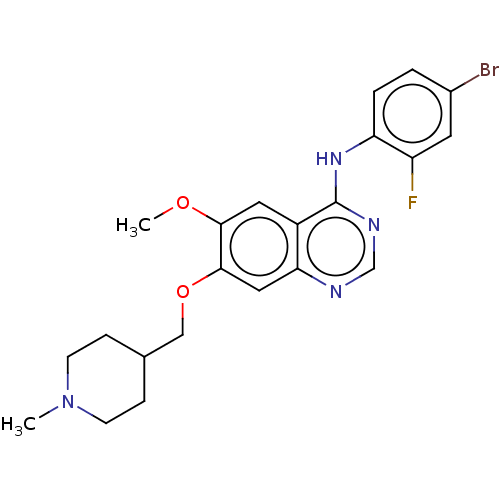 (CHEMBL24828 | N-(4-bromo-2-fluorophenyl)-6-methoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a |
Centre Universitaire Curated by ChEMBL | Assay Description Binding affinity to MER (unknown origin) | Eur J Med Chem 61: 2-25 (2013) Article DOI: 10.1016/j.ejmech.2012.06.005 BindingDB Entry DOI: 10.7270/Q2V40WJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM21079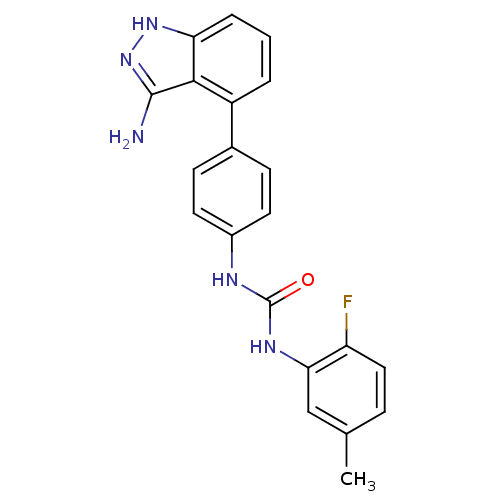 (1-[4-(3-amino-1H-indazol-4-yl)phenyl]-3-(2-fluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q279432J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM31085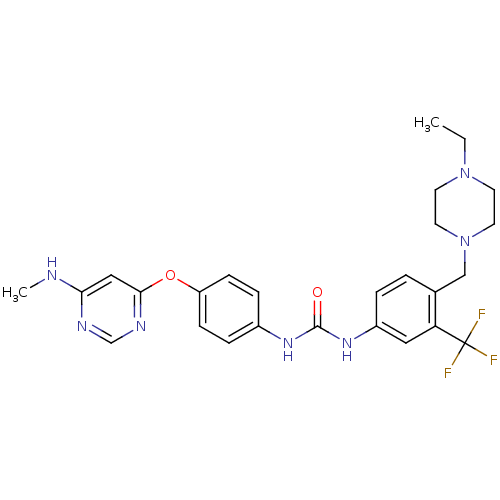 (1-[4-[(4-ethyl-1-piperazinyl)methyl]-3-(trifluorom...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q279432J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM13533 (1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q279432J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM25118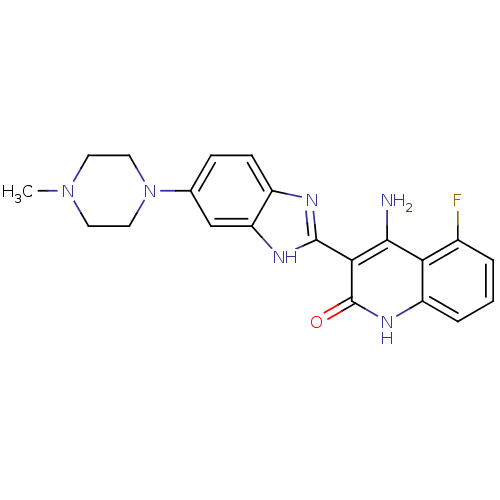 ((3Z)-4-amino-5-fluoro-3-[5-(4-methylpiperazino)-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q279432J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM31090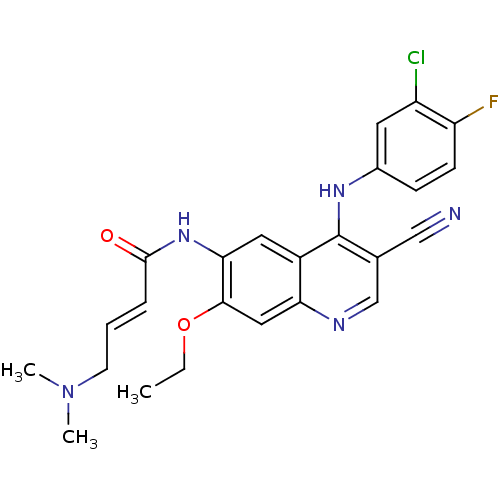 ((E)-N-[4-(3-chloro-4-fluoro-anilino)-3-cyano-7-eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q279432J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM31099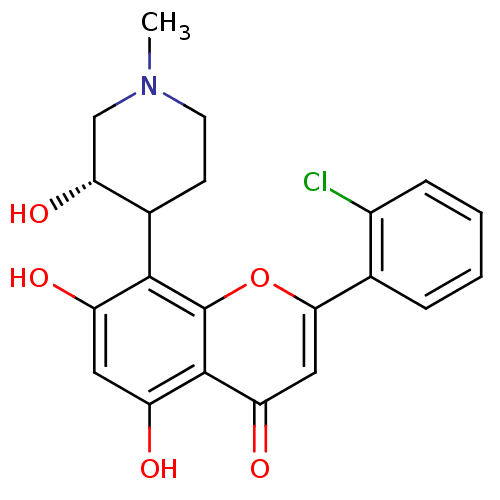 (2-(2-chlorophenyl)-5,7-dihydroxy-8-[(3S)-3-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q279432J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM26474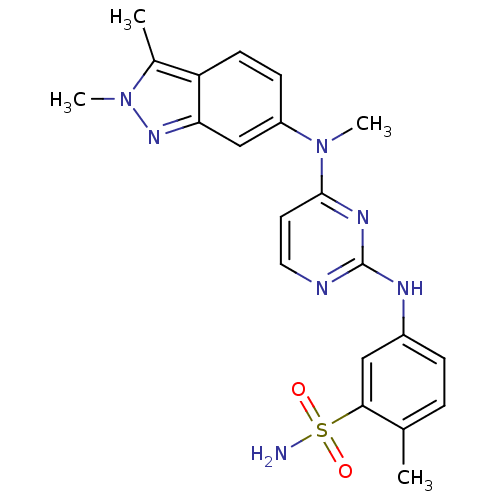 (5-({4-[(2,3-dimethyl-2H-indazol-6-yl)(methyl)amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q279432J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM31093 (4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q279432J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM31094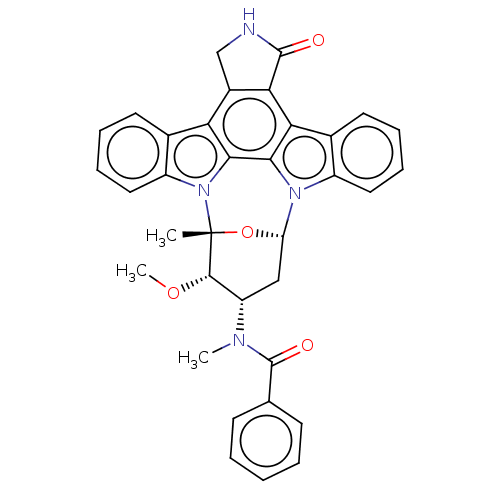 (PKC-412 | cid_24202429) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q279432J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM31095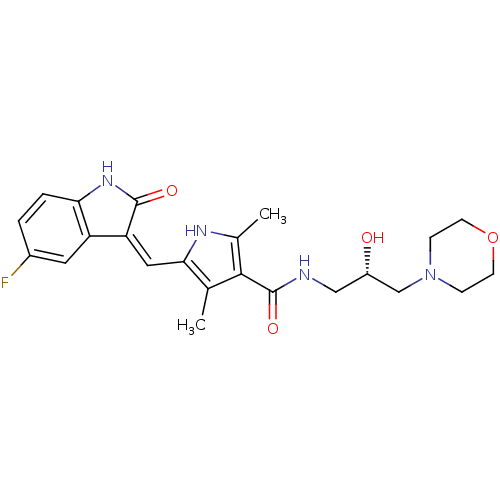 (5-[(Z)-(5-fluoranyl-2-oxidanylidene-1H-indol-3-yli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q279432J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM16673 (4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q279432J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM31096 (CHEMBL290084 | Staurosporine | cid_451705) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q279432J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PCBioAssay | n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q279432J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM21 (CHEMBL24828 | N-(4-bromo-2-fluorophenyl)-6-methoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PCBioAssay | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences Curated by PubChem BioAssay | Assay Description Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q279432J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM350837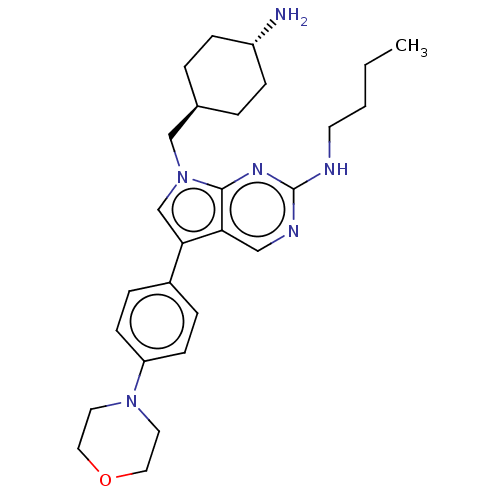 (UNC1532A | US9795606, A1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description The ectopic expression of Mer receptor tyrosine kinase (Mer) has been identified as a tumor cell survival gene product in Acute Lymphoblastic Leukemi... | US Patent US9795606 (2017) BindingDB Entry DOI: 10.7270/Q2FB553D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM350838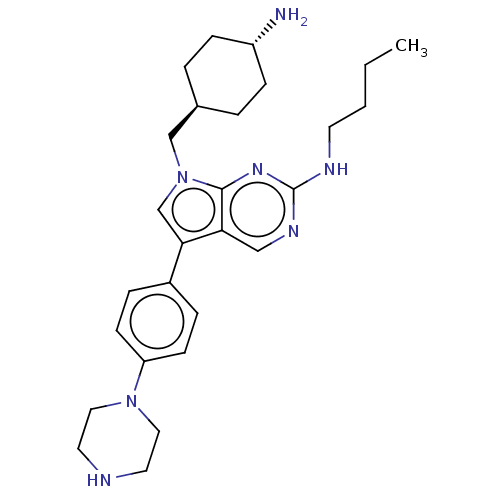 (UNC1533A | US9795606, A2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description The ectopic expression of Mer receptor tyrosine kinase (Mer) has been identified as a tumor cell survival gene product in Acute Lymphoblastic Leukemi... | US Patent US9795606 (2017) BindingDB Entry DOI: 10.7270/Q2FB553D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM350839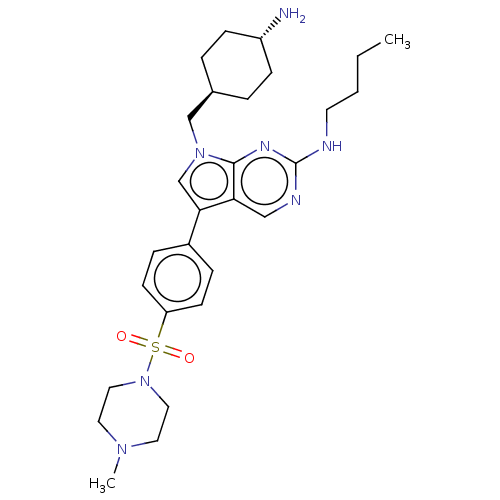 (UNC1534A | US9795606, A3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description The ectopic expression of Mer receptor tyrosine kinase (Mer) has been identified as a tumor cell survival gene product in Acute Lymphoblastic Leukemi... | US Patent US9795606 (2017) BindingDB Entry DOI: 10.7270/Q2FB553D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM350840 (UNC1535A | US9795606, A4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description The ectopic expression of Mer receptor tyrosine kinase (Mer) has been identified as a tumor cell survival gene product in Acute Lymphoblastic Leukemi... | US Patent US9795606 (2017) BindingDB Entry DOI: 10.7270/Q2FB553D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM350841 (UNC1536A | US9795606, A5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description The ectopic expression of Mer receptor tyrosine kinase (Mer) has been identified as a tumor cell survival gene product in Acute Lymphoblastic Leukemi... | US Patent US9795606 (2017) BindingDB Entry DOI: 10.7270/Q2FB553D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM350842 (UNC1970A | US10004755, Compound UNC1970A | US97956...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description The ectopic expression of Mer receptor tyrosine kinase (Mer) has been identified as a tumor cell survival gene product in Acute Lymphoblastic Leukemi... | US Patent US9795606 (2017) BindingDB Entry DOI: 10.7270/Q2FB553D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM350843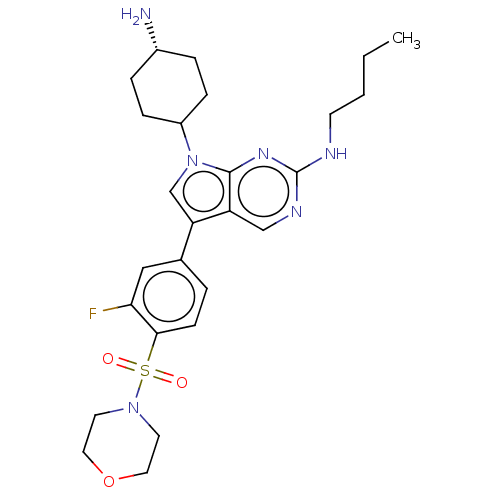 (UNC1971A | US10004755, Compound UNC1971A | US97956...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description The ectopic expression of Mer receptor tyrosine kinase (Mer) has been identified as a tumor cell survival gene product in Acute Lymphoblastic Leukemi... | US Patent US9795606 (2017) BindingDB Entry DOI: 10.7270/Q2FB553D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM350844 (UNC1972A | US10004755, Compound UNC1972A | US97956...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description The ectopic expression of Mer receptor tyrosine kinase (Mer) has been identified as a tumor cell survival gene product in Acute Lymphoblastic Leukemi... | US Patent US9795606 (2017) BindingDB Entry DOI: 10.7270/Q2FB553D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM350845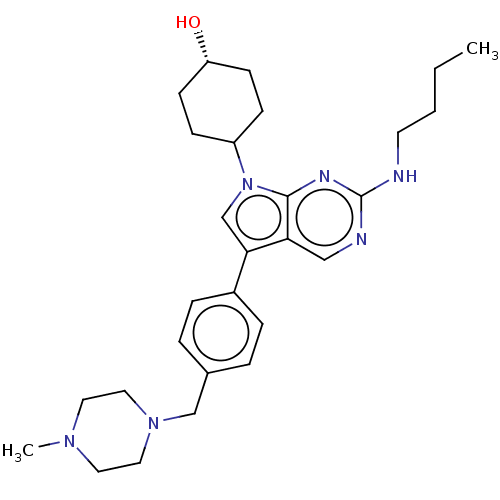 (UNC2025A | US10004755, Compound UNC2025A | US97956...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description The ectopic expression of Mer receptor tyrosine kinase (Mer) has been identified as a tumor cell survival gene product in Acute Lymphoblastic Leukemi... | US Patent US9795606 (2017) BindingDB Entry DOI: 10.7270/Q2FB553D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM350846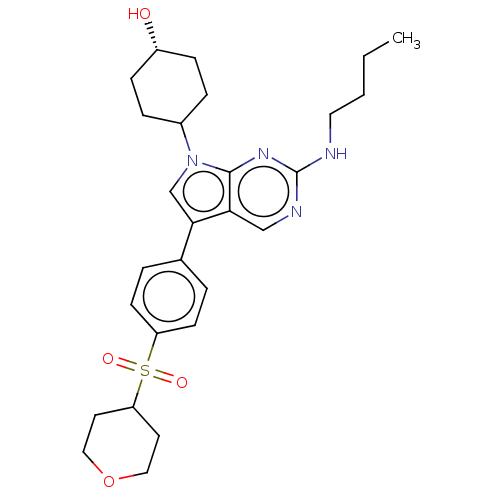 (UNC2026A | US10004755, Compound UNC2026A | US97956...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description The ectopic expression of Mer receptor tyrosine kinase (Mer) has been identified as a tumor cell survival gene product in Acute Lymphoblastic Leukemi... | US Patent US9795606 (2017) BindingDB Entry DOI: 10.7270/Q2FB553D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM350847 (UNC2087A | US10004755, Compound UNC2087A | US97956...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description The ectopic expression of Mer receptor tyrosine kinase (Mer) has been identified as a tumor cell survival gene product in Acute Lymphoblastic Leukemi... | US Patent US9795606 (2017) BindingDB Entry DOI: 10.7270/Q2FB553D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM350848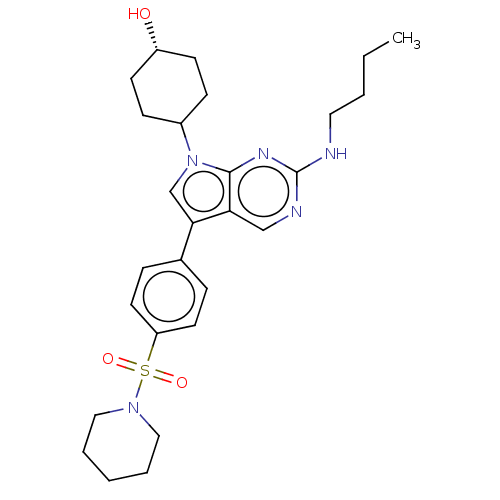 (UNC2078A | US10004755, Compound UNC2078A | US97956...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description The ectopic expression of Mer receptor tyrosine kinase (Mer) has been identified as a tumor cell survival gene product in Acute Lymphoblastic Leukemi... | US Patent US9795606 (2017) BindingDB Entry DOI: 10.7270/Q2FB553D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM350849 (UNC2094A | US10004755, Compound UNC2094A | US97956...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description The ectopic expression of Mer receptor tyrosine kinase (Mer) has been identified as a tumor cell survival gene product in Acute Lymphoblastic Leukemi... | US Patent US9795606 (2017) BindingDB Entry DOI: 10.7270/Q2FB553D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM350850 (UNC2095A | US10004755, Compound UNC2095A | US97956...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description The ectopic expression of Mer receptor tyrosine kinase (Mer) has been identified as a tumor cell survival gene product in Acute Lymphoblastic Leukemi... | US Patent US9795606 (2017) BindingDB Entry DOI: 10.7270/Q2FB553D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM350851 (UNC2123A | US10004755, Compound UNC2123A | US97956...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description The ectopic expression of Mer receptor tyrosine kinase (Mer) has been identified as a tumor cell survival gene product in Acute Lymphoblastic Leukemi... | US Patent US9795606 (2017) BindingDB Entry DOI: 10.7270/Q2FB553D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM350852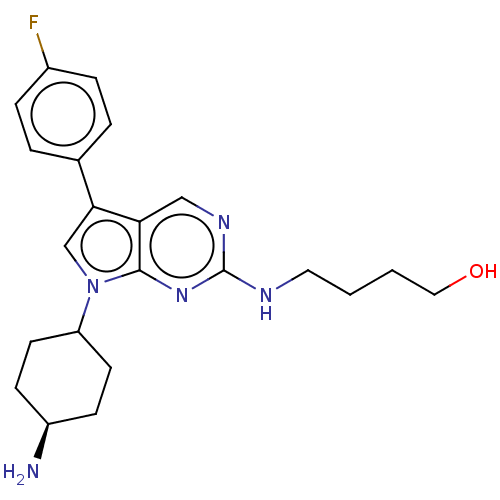 (UNC2124A | US10004755, Compound UNC2124A | US97956...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <250 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description The ectopic expression of Mer receptor tyrosine kinase (Mer) has been identified as a tumor cell survival gene product in Acute Lymphoblastic Leukemi... | US Patent US9795606 (2017) BindingDB Entry DOI: 10.7270/Q2FB553D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM246879 (US10053465, 18 | US10065963, Compound 18 | US10125...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | 25 |
Incyte Corporation US Patent | Assay Description The kinase assay buffer contained 50 mM HEPES, pH7.5, 10 mM MgCl2, 1 mM EGTA, 0.01% NP-40 and 2 mM DTT. 0.1 ul test compounds dissolved in DMSO were ... | US Patent US10053465 (2018) BindingDB Entry DOI: 10.7270/Q2B27X98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM246880 (US10053465, 19 | US10065963, Compound 19 | US10125...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | 25 |
Incyte Corporation US Patent | Assay Description The kinase assay buffer contained 50 mM HEPES, pH7.5, 10 mM MgCl2, 1 mM EGTA, 0.01% NP-40 and 2 mM DTT. 0.1 ul test compounds dissolved in DMSO were ... | US Patent US10053465 (2018) BindingDB Entry DOI: 10.7270/Q2B27X98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4276 total ) | Next | Last >> |