 Found 118 hits Enz. Inhib. hit(s) with Target = 'Mineralocorticoid Receptor (MR)'
Found 118 hits Enz. Inhib. hit(s) with Target = 'Mineralocorticoid Receptor (MR)' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mineralocorticoid receptor [729-984]
(Homo sapiens (Human)) | BDBM238163
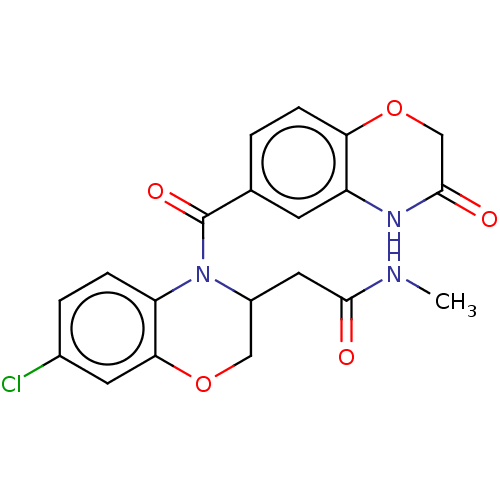
(US10017502, Example 6 | US9394291, 6 | US9394291, ...)Show SMILES CNC(=O)CC1COc2cc(Cl)ccc2N1C(=O)c1ccc2OCC(=O)Nc2c1 Show InChI InChI=1S/C20H18ClN3O5/c1-22-18(25)8-13-9-28-17-7-12(21)3-4-15(17)24(13)20(27)11-2-5-16-14(6-11)23-19(26)10-29-16/h2-7,13H,8-10H2,1H3,(H,22,25)(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB
US Patent
| Assay Description
Test A2: Briefly, in Test A1 the assay was run in 384 well format in 10 mM Tris-HCl, pH 7.5, 0.5 mM EDTA 20 mM NaMoO4, 0.1 mM DTT and 10% glycerol at... |
US Patent US9394291 (2016)
BindingDB Entry DOI: 10.7270/Q2125RJS |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor [729-984]
(Homo sapiens (Human)) | BDBM238159
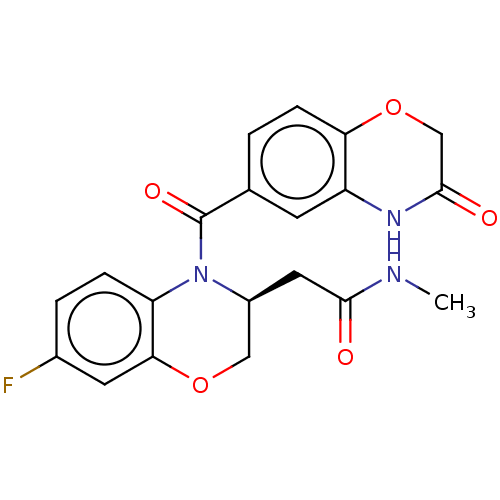
(US10017502, Example 4b | US9394291, 4a)Show SMILES CNC(=O)C[C@H]1COc2cc(F)ccc2N1C(=O)c1ccc2OCC(=O)Nc2c1 |r| Show InChI InChI=1S/C20H18FN3O5/c1-22-18(25)8-13-9-28-17-7-12(21)3-4-15(17)24(13)20(27)11-2-5-16-14(6-11)23-19(26)10-29-16/h2-7,13H,8-10H2,1H3,(H,22,25)(H,23,26)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
US Patent
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB
US Patent
| Assay Description
Test A2: Briefly, in Test A1 the assay was run in 384 well format in 10 mM Tris-HCl, pH 7.5, 0.5 mM EDTA 20 mM NaMoO4, 0.1 mM DTT and 10% glycerol at... |
US Patent US9394291 (2016)
BindingDB Entry DOI: 10.7270/Q2125RJS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mineralocorticoid receptor [729-984]
(Homo sapiens (Human)) | BDBM238151
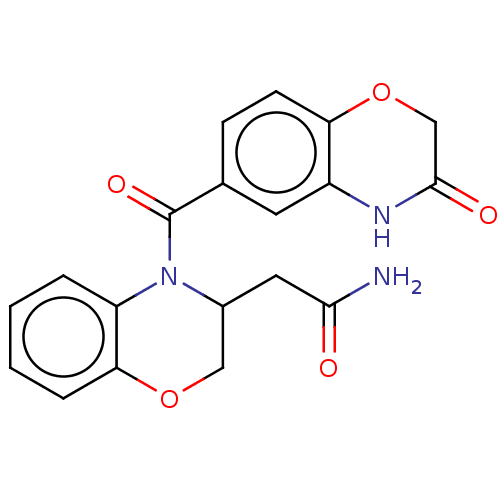
(US10017502, Example 1 | US9394291, 1)Show SMILES NC(=O)CC1COc2ccccc2N1C(=O)c1ccc2OCC(=O)Nc2c1 Show InChI InChI=1S/C19H17N3O5/c20-17(23)8-12-9-26-16-4-2-1-3-14(16)22(12)19(25)11-5-6-15-13(7-11)21-18(24)10-27-15/h1-7,12H,8-10H2,(H2,20,23)(H,21,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB
US Patent
| Assay Description
Test A1: Briefly, in Test A1 the assay was run in 384 well format in 10 mM Tris-HCl, pH 7.5, 0.5 mM EDTA 20 mM NaMoO4, 0.1 mM DTT and 10% glycerol at... |
US Patent US9394291 (2016)
BindingDB Entry DOI: 10.7270/Q2125RJS |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor [729-984]
(Homo sapiens (Human)) | BDBM238152
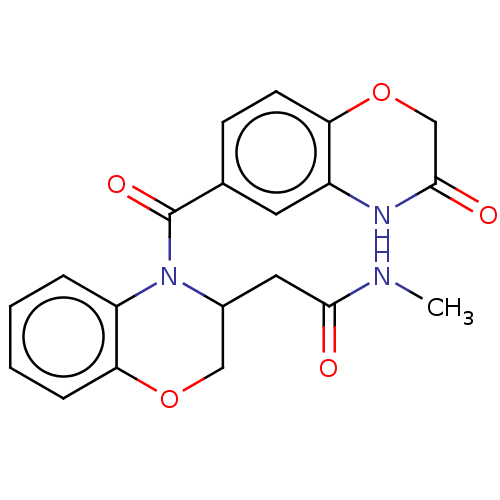
(US10017502, Example 2 | US9394291, 2 | US9394291, ...)Show SMILES CNC(=O)CC1COc2ccccc2N1C(=O)c1ccc2OCC(=O)Nc2c1 Show InChI InChI=1S/C20H19N3O5/c1-21-18(24)9-13-10-27-17-5-3-2-4-15(17)23(13)20(26)12-6-7-16-14(8-12)22-19(25)11-28-16/h2-8,13H,9-11H2,1H3,(H,21,24)(H,22,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB
US Patent
| Assay Description
Test A1: Briefly, in Test A1 the assay was run in 384 well format in 10 mM Tris-HCl, pH 7.5, 0.5 mM EDTA 20 mM NaMoO4, 0.1 mM DTT and 10% glycerol at... |
US Patent US9394291 (2016)
BindingDB Entry DOI: 10.7270/Q2125RJS |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor [729-984]
(Homo sapiens (Human)) | BDBM238155
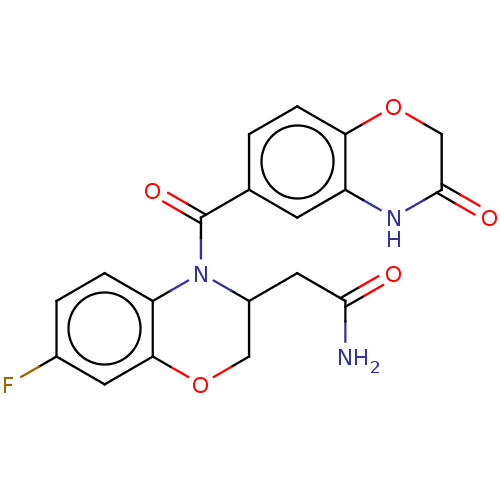
(US10017502, Example 3 | US9394291, 3 | US9394291, ...)Show SMILES NC(=O)CC1COc2cc(F)ccc2N1C(=O)c1ccc2OCC(=O)Nc2c1 Show InChI InChI=1S/C19H16FN3O5/c20-11-2-3-14-16(6-11)27-8-12(7-17(21)24)23(14)19(26)10-1-4-15-13(5-10)22-18(25)9-28-15/h1-6,12H,7-9H2,(H2,21,24)(H,22,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB
US Patent
| Assay Description
Test A1: Briefly, in Test A1 the assay was run in 384 well format in 10 mM Tris-HCl, pH 7.5, 0.5 mM EDTA 20 mM NaMoO4, 0.1 mM DTT and 10% glycerol at... |
US Patent US9394291 (2016)
BindingDB Entry DOI: 10.7270/Q2125RJS |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor [729-984]
(Homo sapiens (Human)) | BDBM238155

(US10017502, Example 3 | US9394291, 3 | US9394291, ...)Show SMILES NC(=O)CC1COc2cc(F)ccc2N1C(=O)c1ccc2OCC(=O)Nc2c1 Show InChI InChI=1S/C19H16FN3O5/c20-11-2-3-14-16(6-11)27-8-12(7-17(21)24)23(14)19(26)10-1-4-15-13(5-10)22-18(25)9-28-15/h1-6,12H,7-9H2,(H2,21,24)(H,22,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB
US Patent
| Assay Description
Test A1: Briefly, in Test A1 the assay was run in 384 well format in 10 mM Tris-HCl, pH 7.5, 0.5 mM EDTA 20 mM NaMoO4, 0.1 mM DTT and 10% glycerol at... |
US Patent US9394291 (2016)
BindingDB Entry DOI: 10.7270/Q2125RJS |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor [729-984]
(Homo sapiens (Human)) | BDBM238158
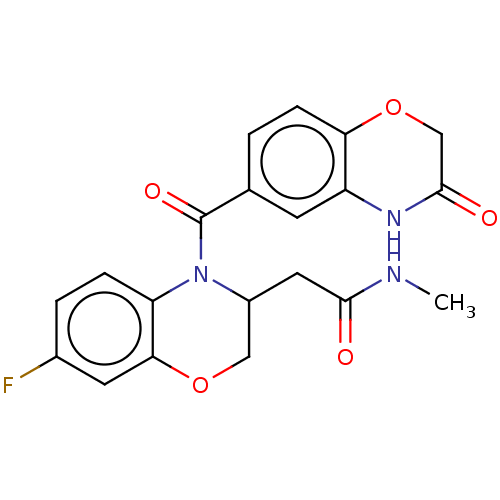
(US10017502, Example 4 | US9394291, 4)Show SMILES CNC(=O)CC1COc2cc(F)ccc2N1C(=O)c1ccc2OCC(=O)Nc2c1 Show InChI InChI=1S/C20H18FN3O5/c1-22-18(25)8-13-9-28-17-7-12(21)3-4-15(17)24(13)20(27)11-2-5-16-14(6-11)23-19(26)10-29-16/h2-7,13H,8-10H2,1H3,(H,22,25)(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
US Patent
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB
US Patent
| Assay Description
Test A1: Briefly, in Test A1 the assay was run in 384 well format in 10 mM Tris-HCl, pH 7.5, 0.5 mM EDTA 20 mM NaMoO4, 0.1 mM DTT and 10% glycerol at... |
US Patent US9394291 (2016)
BindingDB Entry DOI: 10.7270/Q2125RJS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mineralocorticoid receptor [729-984]
(Homo sapiens (Human)) | BDBM238159

(US10017502, Example 4b | US9394291, 4a)Show SMILES CNC(=O)C[C@H]1COc2cc(F)ccc2N1C(=O)c1ccc2OCC(=O)Nc2c1 |r| Show InChI InChI=1S/C20H18FN3O5/c1-22-18(25)8-13-9-28-17-7-12(21)3-4-15(17)24(13)20(27)11-2-5-16-14(6-11)23-19(26)10-29-16/h2-7,13H,8-10H2,1H3,(H,22,25)(H,23,26)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
US Patent
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB
US Patent
| Assay Description
Test A1: Briefly, in Test A1 the assay was run in 384 well format in 10 mM Tris-HCl, pH 7.5, 0.5 mM EDTA 20 mM NaMoO4, 0.1 mM DTT and 10% glycerol at... |
US Patent US9394291 (2016)
BindingDB Entry DOI: 10.7270/Q2125RJS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mineralocorticoid receptor [729-984]
(Homo sapiens (Human)) | BDBM238161

(US10017502, Example 5a | US9394291, 5a)Show SMILES NC(=O)C[C@H]1COc2cc(Cl)ccc2N1C(=O)c1ccc2OCC(=O)Nc2c1 |r| Show InChI InChI=1S/C19H16ClN3O5/c20-11-2-3-14-16(6-11)27-8-12(7-17(21)24)23(14)19(26)10-1-4-15-13(5-10)22-18(25)9-28-15/h1-6,12H,7-9H2,(H2,21,24)(H,22,25)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB
US Patent
| Assay Description
Test A1: Briefly, in Test A1 the assay was run in 384 well format in 10 mM Tris-HCl, pH 7.5, 0.5 mM EDTA 20 mM NaMoO4, 0.1 mM DTT and 10% glycerol at... |
US Patent US9394291 (2016)
BindingDB Entry DOI: 10.7270/Q2125RJS |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor [729-984]
(Homo sapiens (Human)) | BDBM238162
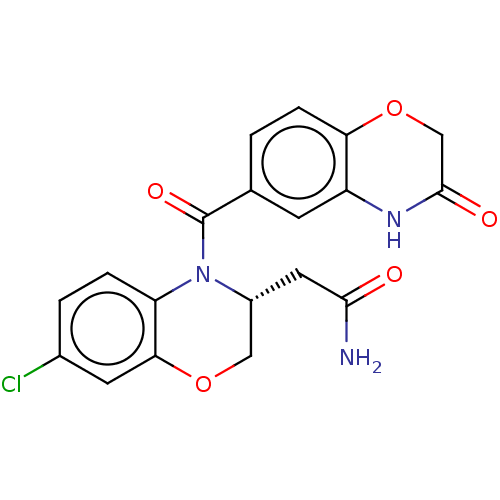
(US10017502, Example 5b | US9394291, 5b)Show SMILES NC(=O)C[C@@H]1COc2cc(Cl)ccc2N1C(=O)c1ccc2OCC(=O)Nc2c1 |r| Show InChI InChI=1S/C19H16ClN3O5/c20-11-2-3-14-16(6-11)27-8-12(7-17(21)24)23(14)19(26)10-1-4-15-13(5-10)22-18(25)9-28-15/h1-6,12H,7-9H2,(H2,21,24)(H,22,25)/t12-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB
US Patent
| Assay Description
Test A1: Briefly, in Test A1 the assay was run in 384 well format in 10 mM Tris-HCl, pH 7.5, 0.5 mM EDTA 20 mM NaMoO4, 0.1 mM DTT and 10% glycerol at... |
US Patent US9394291 (2016)
BindingDB Entry DOI: 10.7270/Q2125RJS |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor [729-984]
(Homo sapiens (Human)) | BDBM238163

(US10017502, Example 6 | US9394291, 6 | US9394291, ...)Show SMILES CNC(=O)CC1COc2cc(Cl)ccc2N1C(=O)c1ccc2OCC(=O)Nc2c1 Show InChI InChI=1S/C20H18ClN3O5/c1-22-18(25)8-13-9-28-17-7-12(21)3-4-15(17)24(13)20(27)11-2-5-16-14(6-11)23-19(26)10-29-16/h2-7,13H,8-10H2,1H3,(H,22,25)(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB
US Patent
| Assay Description
Test A1: Briefly, in Test A1 the assay was run in 384 well format in 10 mM Tris-HCl, pH 7.5, 0.5 mM EDTA 20 mM NaMoO4, 0.1 mM DTT and 10% glycerol at... |
US Patent US9394291 (2016)
BindingDB Entry DOI: 10.7270/Q2125RJS |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor [729-984]
(Homo sapiens (Human)) | BDBM238163

(US10017502, Example 6 | US9394291, 6 | US9394291, ...)Show SMILES CNC(=O)CC1COc2cc(Cl)ccc2N1C(=O)c1ccc2OCC(=O)Nc2c1 Show InChI InChI=1S/C20H18ClN3O5/c1-22-18(25)8-13-9-28-17-7-12(21)3-4-15(17)24(13)20(27)11-2-5-16-14(6-11)23-19(26)10-29-16/h2-7,13H,8-10H2,1H3,(H,22,25)(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB
US Patent
| Assay Description
Test A1: Briefly, in Test A1 the assay was run in 384 well format in 10 mM Tris-HCl, pH 7.5, 0.5 mM EDTA 20 mM NaMoO4, 0.1 mM DTT and 10% glycerol at... |
US Patent US9394291 (2016)
BindingDB Entry DOI: 10.7270/Q2125RJS |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor [729-984]
(Homo sapiens (Human)) | BDBM238163

(US10017502, Example 6 | US9394291, 6 | US9394291, ...)Show SMILES CNC(=O)CC1COc2cc(Cl)ccc2N1C(=O)c1ccc2OCC(=O)Nc2c1 Show InChI InChI=1S/C20H18ClN3O5/c1-22-18(25)8-13-9-28-17-7-12(21)3-4-15(17)24(13)20(27)11-2-5-16-14(6-11)23-19(26)10-29-16/h2-7,13H,8-10H2,1H3,(H,22,25)(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB
US Patent
| Assay Description
Test A1: Briefly, in Test A1 the assay was run in 384 well format in 10 mM Tris-HCl, pH 7.5, 0.5 mM EDTA 20 mM NaMoO4, 0.1 mM DTT and 10% glycerol at... |
US Patent US9394291 (2016)
BindingDB Entry DOI: 10.7270/Q2125RJS |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor [729-984]
(Homo sapiens (Human)) | BDBM238166
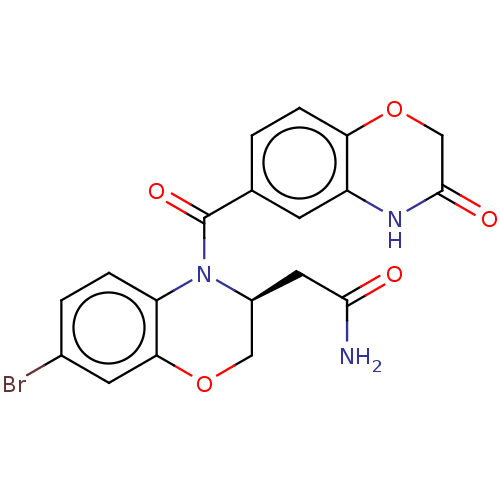
(US10017502, Example 7 | US9394291, 7)Show SMILES NC(=O)C[C@H]1COc2cc(Br)ccc2N1C(=O)c1ccc2OCC(=O)Nc2c1 |r| Show InChI InChI=1S/C19H16BrN3O5/c20-11-2-3-14-16(6-11)27-8-12(7-17(21)24)23(14)19(26)10-1-4-15-13(5-10)22-18(25)9-28-15/h1-6,12H,7-9H2,(H2,21,24)(H,22,25)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB
US Patent
| Assay Description
Test A1: Briefly, in Test A1 the assay was run in 384 well format in 10 mM Tris-HCl, pH 7.5, 0.5 mM EDTA 20 mM NaMoO4, 0.1 mM DTT and 10% glycerol at... |
US Patent US9394291 (2016)
BindingDB Entry DOI: 10.7270/Q2125RJS |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor [729-984]
(Homo sapiens (Human)) | BDBM238167

(US10017502, Example 8 | US9394291, 8 | US9394291, ...)Show SMILES CNC(=O)CC1COc2cc(Br)ccc2N1C(=O)c1ccc2OCC(=O)Nc2c1 Show InChI InChI=1S/C20H18BrN3O5/c1-22-18(25)8-13-9-28-17-7-12(21)3-4-15(17)24(13)20(27)11-2-5-16-14(6-11)23-19(26)10-29-16/h2-7,13H,8-10H2,1H3,(H,22,25)(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB
US Patent
| Assay Description
Test A1: Briefly, in Test A1 the assay was run in 384 well format in 10 mM Tris-HCl, pH 7.5, 0.5 mM EDTA 20 mM NaMoO4, 0.1 mM DTT and 10% glycerol at... |
US Patent US9394291 (2016)
BindingDB Entry DOI: 10.7270/Q2125RJS |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor [729-984]
(Homo sapiens (Human)) | BDBM238167

(US10017502, Example 8 | US9394291, 8 | US9394291, ...)Show SMILES CNC(=O)CC1COc2cc(Br)ccc2N1C(=O)c1ccc2OCC(=O)Nc2c1 Show InChI InChI=1S/C20H18BrN3O5/c1-22-18(25)8-13-9-28-17-7-12(21)3-4-15(17)24(13)20(27)11-2-5-16-14(6-11)23-19(26)10-29-16/h2-7,13H,8-10H2,1H3,(H,22,25)(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB
US Patent
| Assay Description
Test A1: Briefly, in Test A1 the assay was run in 384 well format in 10 mM Tris-HCl, pH 7.5, 0.5 mM EDTA 20 mM NaMoO4, 0.1 mM DTT and 10% glycerol at... |
US Patent US9394291 (2016)
BindingDB Entry DOI: 10.7270/Q2125RJS |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor [729-984]
(Homo sapiens (Human)) | BDBM238167

(US10017502, Example 8 | US9394291, 8 | US9394291, ...)Show SMILES CNC(=O)CC1COc2cc(Br)ccc2N1C(=O)c1ccc2OCC(=O)Nc2c1 Show InChI InChI=1S/C20H18BrN3O5/c1-22-18(25)8-13-9-28-17-7-12(21)3-4-15(17)24(13)20(27)11-2-5-16-14(6-11)23-19(26)10-29-16/h2-7,13H,8-10H2,1H3,(H,22,25)(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB
US Patent
| Assay Description
Test A1: Briefly, in Test A1 the assay was run in 384 well format in 10 mM Tris-HCl, pH 7.5, 0.5 mM EDTA 20 mM NaMoO4, 0.1 mM DTT and 10% glycerol at... |
US Patent US9394291 (2016)
BindingDB Entry DOI: 10.7270/Q2125RJS |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor [729-984]
(Homo sapiens (Human)) | BDBM238151

(US10017502, Example 1 | US9394291, 1)Show SMILES NC(=O)CC1COc2ccccc2N1C(=O)c1ccc2OCC(=O)Nc2c1 Show InChI InChI=1S/C19H17N3O5/c20-17(23)8-12-9-26-16-4-2-1-3-14(16)22(12)19(25)11-5-6-15-13(7-11)21-18(24)10-27-15/h1-7,12H,8-10H2,(H2,20,23)(H,21,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB
US Patent
| Assay Description
Test A2: Briefly, in Test A1 the assay was run in 384 well format in 10 mM Tris-HCl, pH 7.5, 0.5 mM EDTA 20 mM NaMoO4, 0.1 mM DTT and 10% glycerol at... |
US Patent US9394291 (2016)
BindingDB Entry DOI: 10.7270/Q2125RJS |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor [729-984]
(Homo sapiens (Human)) | BDBM238152

(US10017502, Example 2 | US9394291, 2 | US9394291, ...)Show SMILES CNC(=O)CC1COc2ccccc2N1C(=O)c1ccc2OCC(=O)Nc2c1 Show InChI InChI=1S/C20H19N3O5/c1-21-18(24)9-13-10-27-17-5-3-2-4-15(17)23(13)20(26)12-6-7-16-14(8-12)22-19(25)11-28-16/h2-8,13H,9-11H2,1H3,(H,21,24)(H,22,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB
US Patent
| Assay Description
Test A2: Briefly, in Test A1 the assay was run in 384 well format in 10 mM Tris-HCl, pH 7.5, 0.5 mM EDTA 20 mM NaMoO4, 0.1 mM DTT and 10% glycerol at... |
US Patent US9394291 (2016)
BindingDB Entry DOI: 10.7270/Q2125RJS |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor [729-984]
(Homo sapiens (Human)) | BDBM238158

(US10017502, Example 4 | US9394291, 4)Show SMILES CNC(=O)CC1COc2cc(F)ccc2N1C(=O)c1ccc2OCC(=O)Nc2c1 Show InChI InChI=1S/C20H18FN3O5/c1-22-18(25)8-13-9-28-17-7-12(21)3-4-15(17)24(13)20(27)11-2-5-16-14(6-11)23-19(26)10-29-16/h2-7,13H,8-10H2,1H3,(H,22,25)(H,23,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
US Patent
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | 7.5 | n/a |
AstraZeneca AB
US Patent
| Assay Description
Test A2: Briefly, in Test A1 the assay was run in 384 well format in 10 mM Tris-HCl, pH 7.5, 0.5 mM EDTA 20 mM NaMoO4, 0.1 mM DTT and 10% glycerol at... |
US Patent US9394291 (2016)
BindingDB Entry DOI: 10.7270/Q2125RJS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mineralocorticoid receptor
(RAT) | BDBM50004519

(CHEMBL2181929)Show SMILES CC1(C)Oc2cc(NS(C)(=O)=O)ccc2N(C1=O)c1ccc(F)cc1 Show InChI InChI=1S/C17H17FN2O4S/c1-17(2)16(21)20(13-7-4-11(18)5-8-13)14-9-6-12(10-15(14)24-17)19-25(3,22)23/h4-10,19H,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50318300
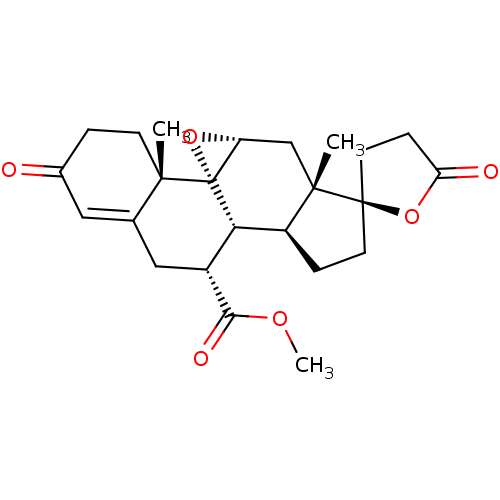
(CHEMBL1095097 | EPLERENONE | SC-66110)Show SMILES COC(=O)[C@@H]1CC2=CC(=O)CC[C@]2(C)[C@@]23O[C@@H]2C[C@@]2(C)[C@@H](CC[C@@]22CCC(=O)O2)[C@H]13 |r,t:6| Show InChI InChI=1S/C24H30O6/c1-21-7-4-14(25)10-13(21)11-15(20(27)28-3)19-16-5-8-23(9-6-18(26)30-23)22(16,2)12-17-24(19,21)29-17/h10,15-17,19H,4-9,11-12H2,1-3H3/t15-,16+,17-,19+,21+,22+,23-,24-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mineralocorticoid receptor
(RAT) | BDBM50597084

(CHEMBL5174401)Show SMILES CC1Oc2cc(NS(C)(=O)=O)ccc2N(C1=O)c1ccc(F)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 201 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597083
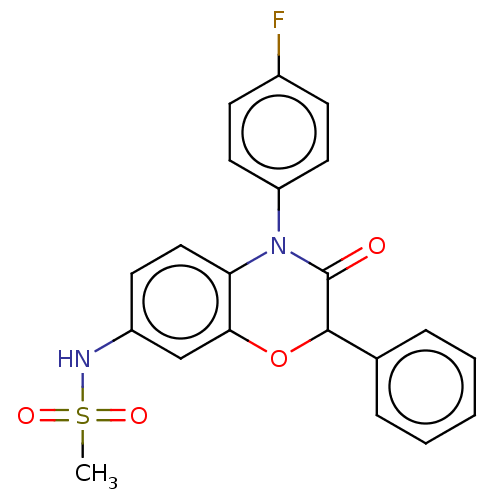
(CHEMBL5171957)Show SMILES CS(=O)(=O)Nc1ccc2N(C(=O)C(Oc2c1)c1ccccc1)c1ccc(F)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597078

(CHEMBL5181002) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 357 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597087
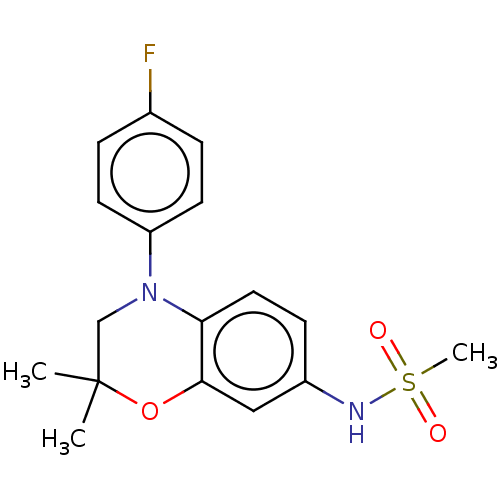
(CHEMBL5181278)Show SMILES CC1(C)CN(c2ccc(F)cc2)c2ccc(NS(C)(=O)=O)cc2O1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM86690

(A 348441 | A-348441)Show SMILES CC#C[C@]1(O)CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3[C@H](C[C@]12C)c1ccc(cc1)N(C)CCO[C@H]1CCC2C(C1)C[C@@H](O)C1C3CCC(C(C)CCC(O)=O)C3[C@@H](O)CC21 |r,c:18,t:11| Show InChI InChI=1S/C52H71NO7/c1-5-21-52(59)22-20-44-40-14-9-32-25-35(54)12-15-39(32)48(40)43(29-51(44,52)3)31-7-10-34(11-8-31)53(4)23-24-60-36-13-16-38-33(26-36)27-45(55)50-41-18-17-37(30(2)6-19-47(57)58)49(41)46(56)28-42(38)50/h7-8,10-11,25,30,33,36-38,40-46,49-50,55-56,59H,6,9,12-20,22-24,26-29H2,1-4H3,(H,57,58)/t30?,33?,36-,37?,38?,40-,41?,42?,43+,44-,45+,46-,49?,50?,51-,52-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 191-200 (2005)
Article DOI: 10.1124/jpet.104.081257
BindingDB Entry DOI: 10.7270/Q2FT8J68 |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597085
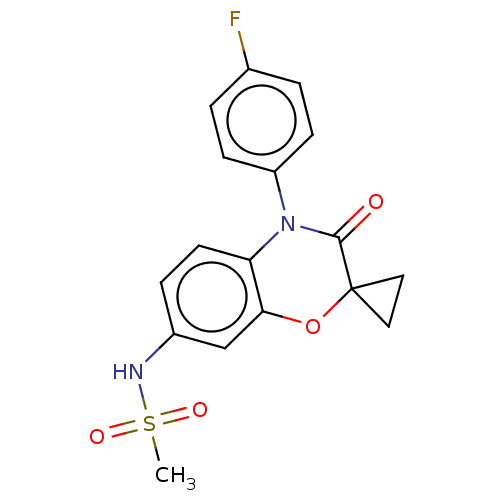
(CHEMBL5183454)Show SMILES CS(=O)(=O)Nc1ccc2N(C(=O)C3(CC3)Oc2c1)c1ccc(F)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 549 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597081
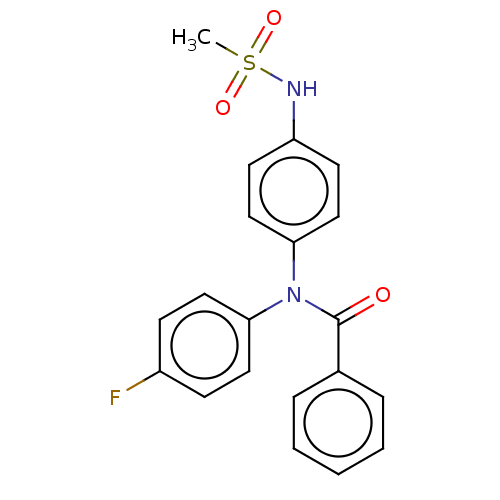
(CHEMBL5184647)Show SMILES CS(=O)(=O)Nc1ccc(cc1)N(C(=O)c1ccccc1)c1ccc(F)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 596 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597086
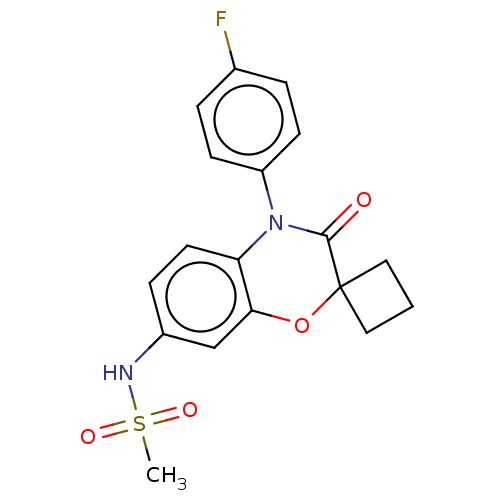
(CHEMBL5187656)Show SMILES CS(=O)(=O)Nc1ccc2N(C(=O)C3(CCC3)Oc2c1)c1ccc(F)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 626 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM86689
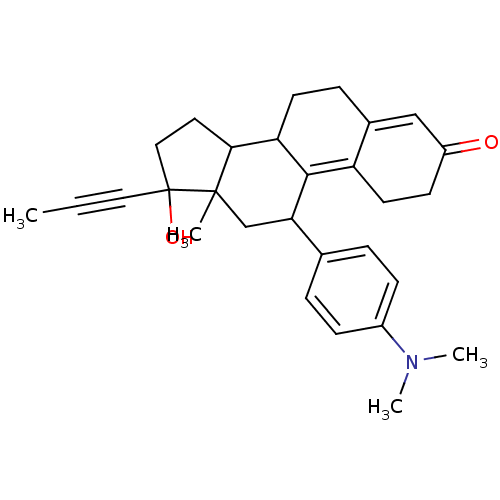
(CAS_84371-65-3 | NSC_55245 | RU-486)Show SMILES CC#CC1(O)CCC2C3CCC4=CC(=O)CCC4=C3C(CC12C)c1ccc(cc1)N(C)C |c:18,t:11| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 191-200 (2005)
Article DOI: 10.1124/jpet.104.081257
BindingDB Entry DOI: 10.7270/Q2FT8J68 |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597076

(CHEMBL5176336) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 853 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597080
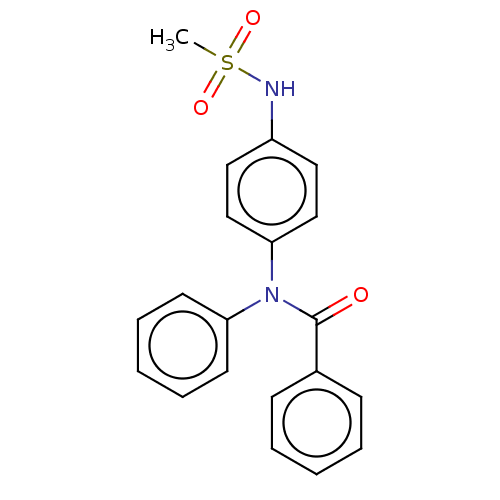
(CHEMBL5207249)Show SMILES CS(=O)(=O)Nc1ccc(cc1)N(C(=O)c1ccccc1)c1ccccc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597077
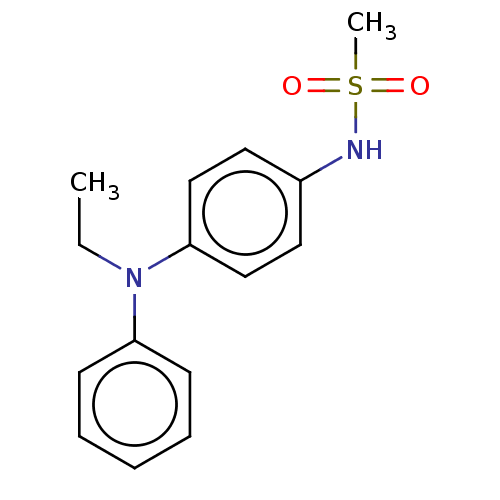
(CHEMBL5172350) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597082

(CHEMBL5204612) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597068
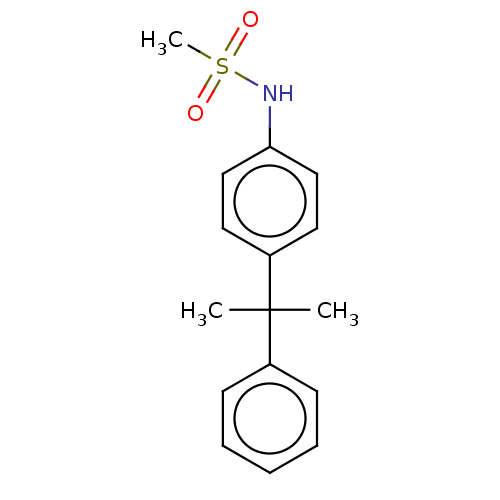
(CHEMBL5201004) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597070
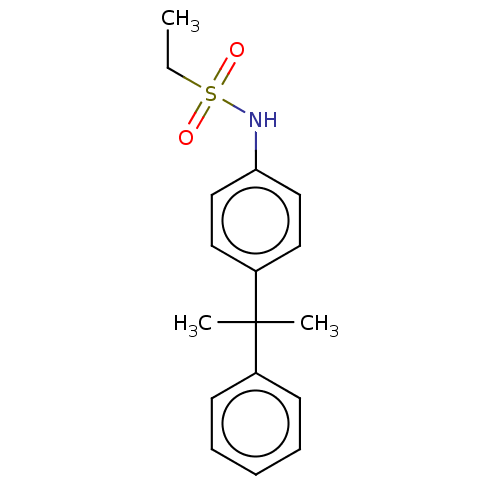
(CHEMBL5186524) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597073
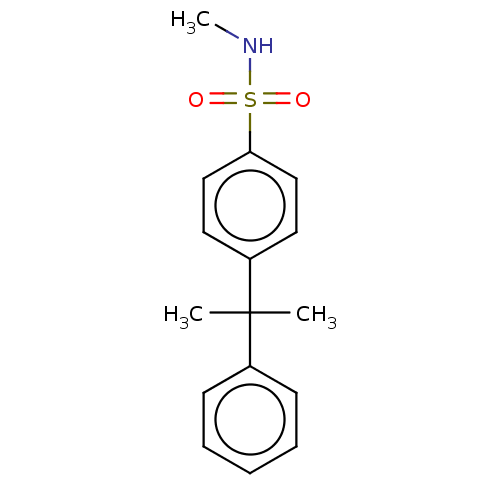
(CHEMBL5182687) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597079
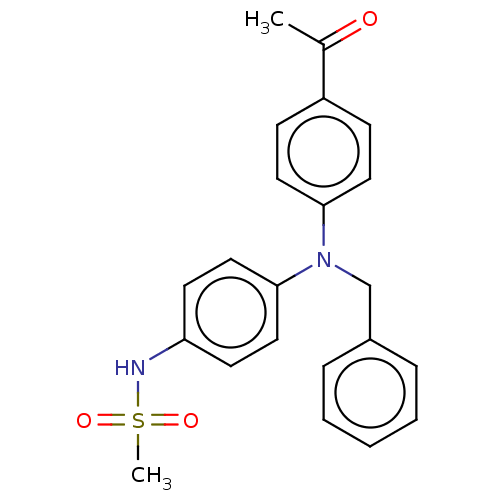
(CHEMBL5207123)Show SMILES CC(=O)c1ccc(cc1)N(Cc1ccccc1)c1ccc(NS(C)(=O)=O)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597071
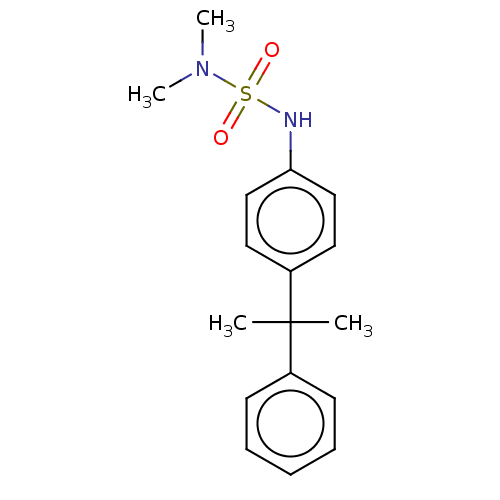
(CHEMBL5191961) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597074

(CHEMBL5197264) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597075
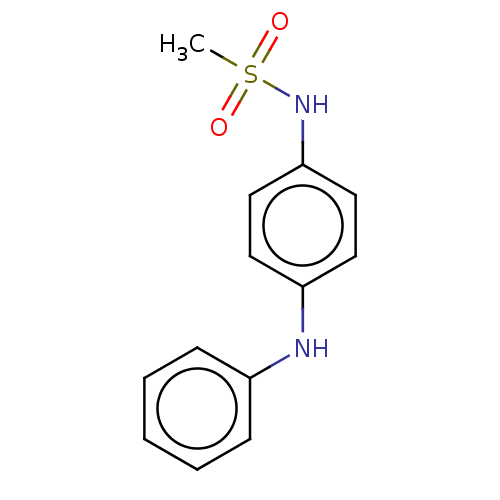
(CHEMBL5186231) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597069

(CHEMBL5208644)Show SMILES CC(C)(c1ccccc1)c1ccc(NS(=O)(=O)C(F)(F)F)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597067

(CHEMBL5182675) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM50597072

(CHEMBL5173089) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00402
BindingDB Entry DOI: 10.7270/Q2571H1M |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM19191

((R)-[(4aR,5S)-1-(4-fluorophenyl)-4a-methyl-1H,4H,4...)Show SMILES [H][C@@]1(CCCC2=Cc3c(C[C@]12C)cnn3-c1ccc(F)cc1)[C@@H](O)c1ccccc1 |r,t:5| Show InChI InChI=1S/C25H25FN2O/c1-25-15-18-16-27-28(21-12-10-20(26)11-13-21)23(18)14-19(25)8-5-9-22(25)24(29)17-6-3-2-4-7-17/h2-4,6-7,10-14,16,22,24,29H,5,8-9,15H2,1H3/t22-,24+,25+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
The IC50s were determined by incubating the receptors with radiolabeled aldosterone in the presence of full log scale concentrations (10-11 M to 10-6... |
J Med Chem 47: 2441-52 (2004)
Article DOI: 10.1021/jm030585i
BindingDB Entry DOI: 10.7270/Q28W3BKZ |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM19192
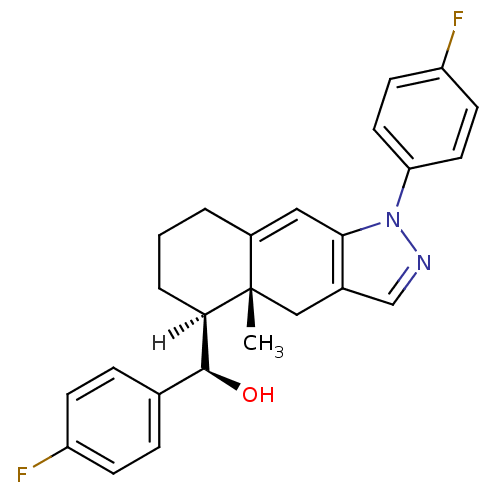
((R)-[(4aR,5S)-1-(4-fluorophenyl)-4a-methyl-1H,4H,4...)Show SMILES [H][C@@]1(CCCC2=Cc3c(C[C@]12C)cnn3-c1ccc(F)cc1)[C@@H](O)c1ccc(F)cc1 |r,t:5| Show InChI InChI=1S/C25H24F2N2O/c1-25-14-17-15-28-29(21-11-9-20(27)10-12-21)23(17)13-18(25)3-2-4-22(25)24(30)16-5-7-19(26)8-6-16/h5-13,15,22,24,30H,2-4,14H2,1H3/t22-,24+,25+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
The IC50s were determined by incubating the receptors with radiolabeled aldosterone in the presence of full log scale concentrations (10-11 M to 10-6... |
J Med Chem 47: 2441-52 (2004)
Article DOI: 10.1021/jm030585i
BindingDB Entry DOI: 10.7270/Q28W3BKZ |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM19199
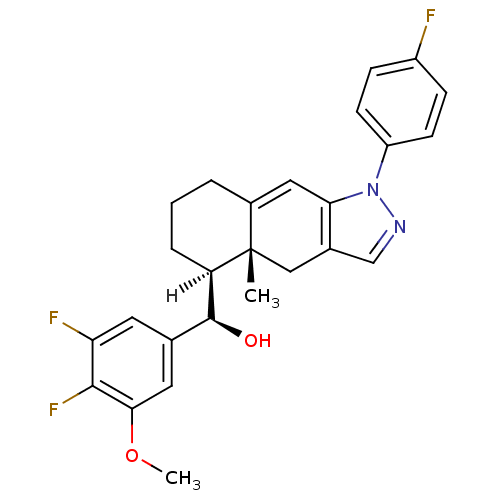
((R)-[(4aR,5S)-1-(4-fluorophenyl)-4a-methyl-1H,4H,4...)Show SMILES [H][C@@]1(CCCC2=Cc3c(C[C@]12C)cnn3-c1ccc(F)cc1)[C@@H](O)c1cc(F)c(F)c(OC)c1 |r,t:5| Show InChI InChI=1S/C26H25F3N2O2/c1-26-13-16-14-30-31(19-8-6-18(27)7-9-19)22(16)12-17(26)4-3-5-20(26)25(32)15-10-21(28)24(29)23(11-15)33-2/h6-12,14,20,25,32H,3-5,13H2,1-2H3/t20-,25+,26+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
The IC50s were determined by incubating the receptors with radiolabeled aldosterone in the presence of full log scale concentrations (10-11 M to 10-6... |
J Med Chem 47: 2441-52 (2004)
Article DOI: 10.1021/jm030585i
BindingDB Entry DOI: 10.7270/Q28W3BKZ |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(RAT) | BDBM19200
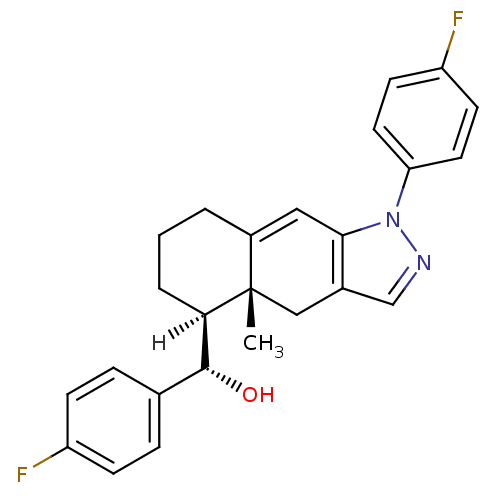
((S)-[(4aR,5S)-1-(4-fluorophenyl)-4a-methyl-1H,4H,4...)Show SMILES [H][C@@]1(CCCC2=Cc3c(C[C@]12C)cnn3-c1ccc(F)cc1)[C@H](O)c1ccc(F)cc1 |r,t:5| Show InChI InChI=1S/C25H24F2N2O/c1-25-14-17-15-28-29(21-11-9-20(27)10-12-21)23(17)13-18(25)3-2-4-22(25)24(30)16-5-7-19(26)8-6-16/h5-13,15,22,24,30H,2-4,14H2,1H3/t22-,24-,25+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
The IC50s were determined by incubating the receptors with radiolabeled aldosterone in the presence of full log scale concentrations (10-11 M to 10-6... |
J Med Chem 47: 2441-52 (2004)
Article DOI: 10.1021/jm030585i
BindingDB Entry DOI: 10.7270/Q28W3BKZ |
More data for this
Ligand-Target Pair | |
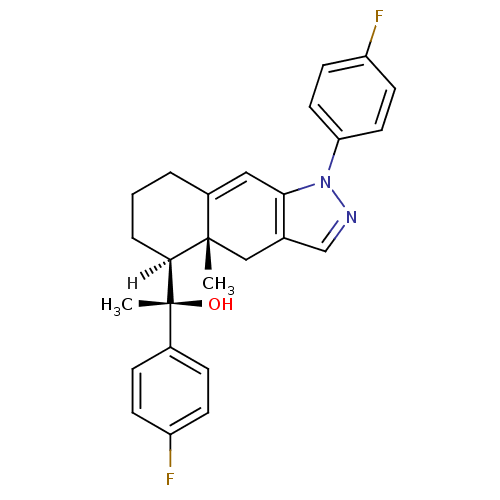
Mineralocorticoid receptor
(RAT) | BDBM19201
((1S)-1-[(4aR,5S)-1-(4-fluorophenyl)-4a-methyl-1H,4...)Show SMILES [H][C@@]1(CCCC2=Cc3c(C[C@]12C)cnn3-c1ccc(F)cc1)[C@](C)(O)c1ccc(F)cc1 |r,t:5| Show InChI InChI=1S/C26H26F2N2O/c1-25-15-17-16-29-30(22-12-10-21(28)11-13-22)23(17)14-19(25)4-3-5-24(25)26(2,31)18-6-8-20(27)9-7-18/h6-14,16,24,31H,3-5,15H2,1-2H3/t24-,25-,26+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
The IC50s were determined by incubating the receptors with radiolabeled aldosterone in the presence of full log scale concentrations (10-11 M to 10-6... |
J Med Chem 47: 2441-52 (2004)
Article DOI: 10.1021/jm030585i
BindingDB Entry DOI: 10.7270/Q28W3BKZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data