

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50151008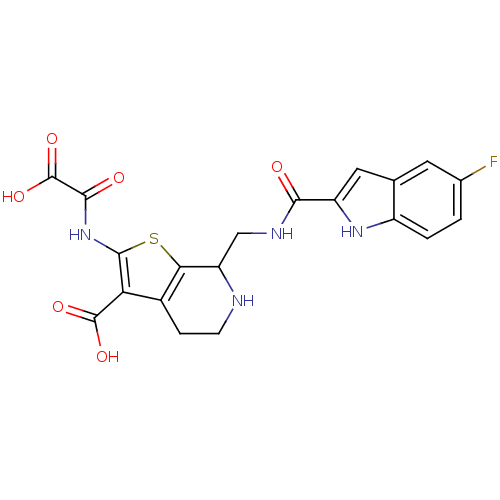 ((7S)-2-[(carboxycarbonyl)amino]-7-({[(5-fluoro-1H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Serono Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Protein-tyrosine phosphatase 1B at pH 5.5 | J Med Chem 47: 4142-6 (2004) Article DOI: 10.1021/jm030629n BindingDB Entry DOI: 10.7270/Q2571BGC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118750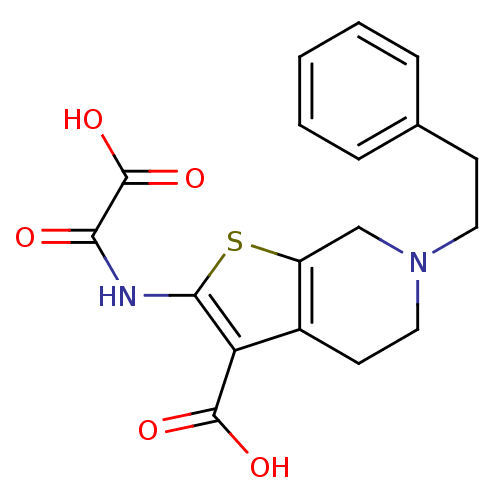 (2-(Oxalyl-amino)-6-phenethyl-4,5,6,7-tetrahydro-th...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118750 (2-(Oxalyl-amino)-6-phenethyl-4,5,6,7-tetrahydro-th...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B (1 to 321 residues) catalytic domain expressed in Escherichia coli BL21 (DE3) cells using p-nitrophenyl phospha... | Bioorg Med Chem 24: 3343-52 (2016) Article DOI: 10.1016/j.bmc.2016.06.035 BindingDB Entry DOI: 10.7270/Q20Z756P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118792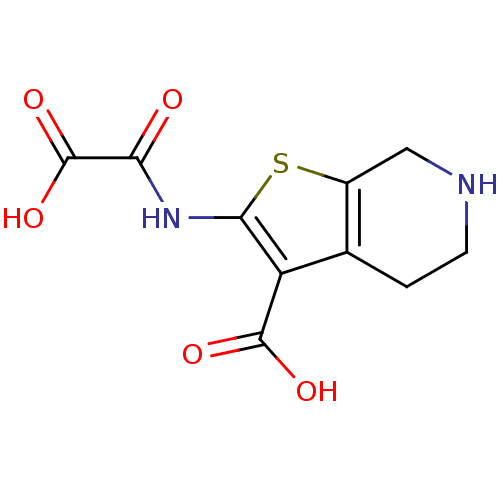 (2-(OXALYL-AMINO)-4,5,6,7-TETRAHYDRO-THIENO[2,3-C]P...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118786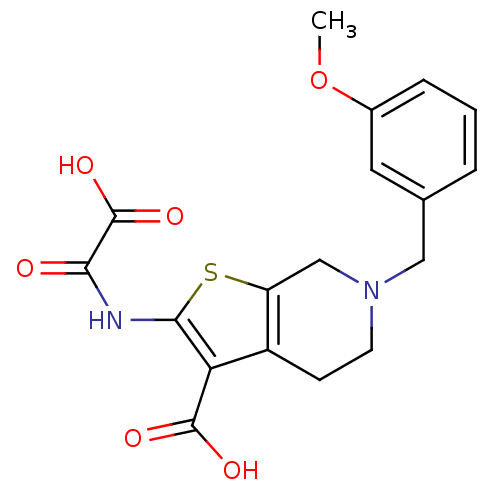 (6-(3-Methoxy-benzyl)-2-(oxalyl-amino)-4,5,6,7-tetr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118794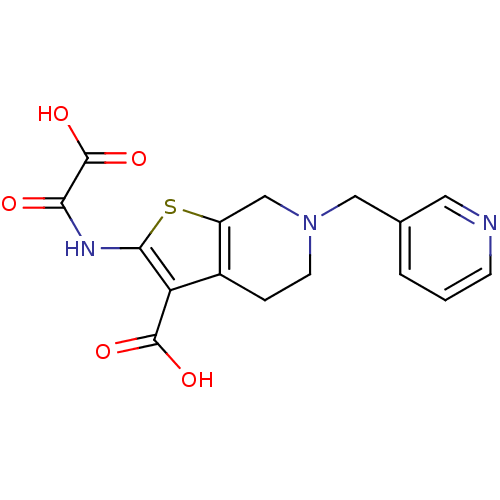 (2-(Oxalyl-amino)-6-pyridin-3-ylmethyl-4,5,6,7-tetr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118793 (6-Methyl-2-(oxalyl-amino)-4,5,6,7-tetrahydro-thien...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118774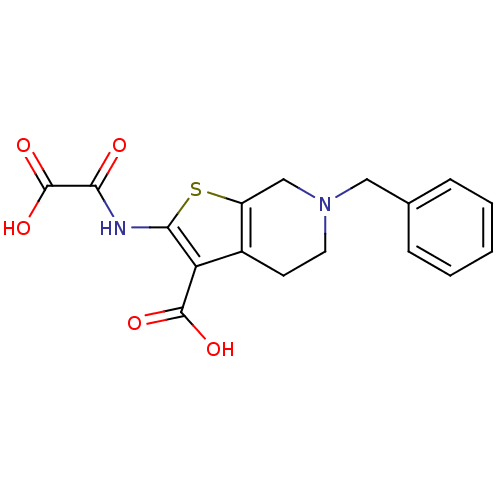 (6-Benzyl-2-(oxalyl-amino)-4,5,6,7-tetrahydro-thien...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118742 (2-(Oxalyl-amino)-6-pyridin-2-ylmethyl-4,5,6,7-tetr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118755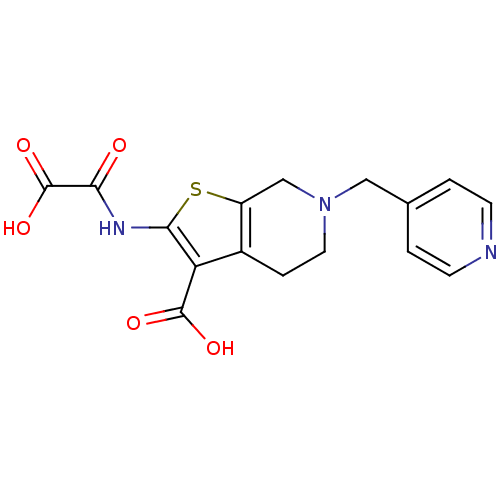 (2-(Oxalyl-amino)-6-pyridin-4-ylmethyl-4,5,6,7-tetr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118772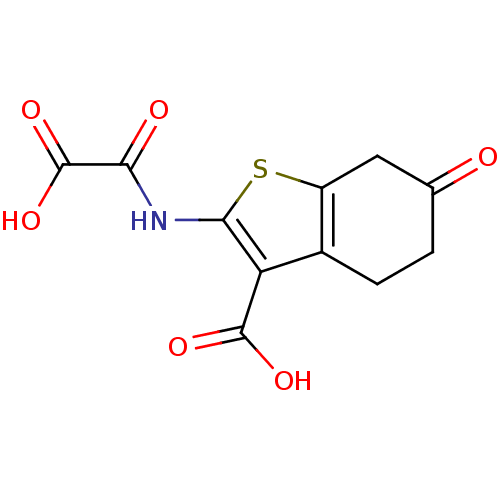 (2-(Oxalyl-amino)-6-oxo-4,5,6,7-tetrahydro-benzo[b]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118767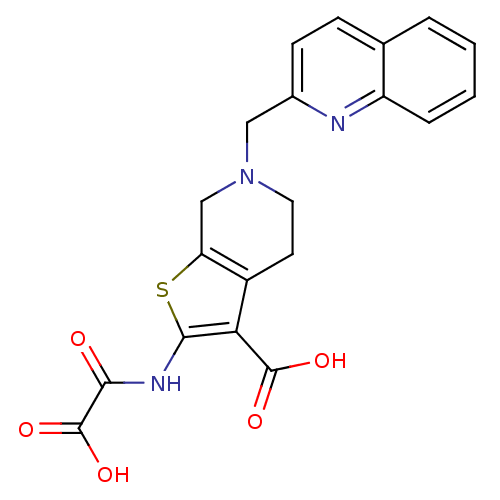 (2-(Oxalyl-amino)-6-quinolin-2-ylmethyl-4,5,6,7-tet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118771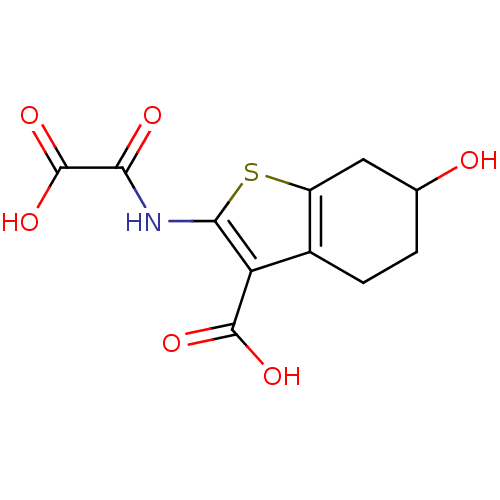 (2-(carboxyformamido)-6-hydroxy-4,5,6,7-tetrahydrob...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118741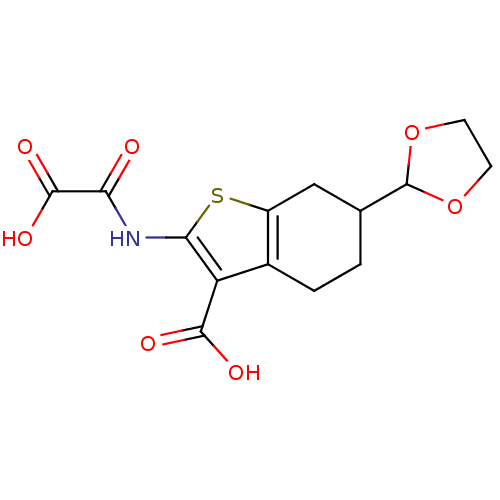 (6-[1,3]Dioxolan-2-yl-2-(oxalyl-amino)-4,5,6,7-tetr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118765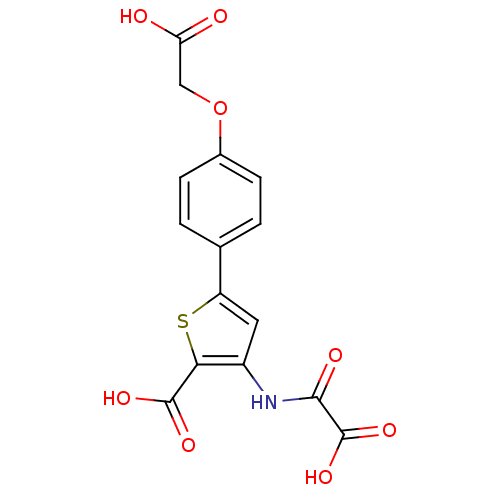 (3-(carboxyformamido)-5-(4-(carboxymethoxy)phenyl)t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118782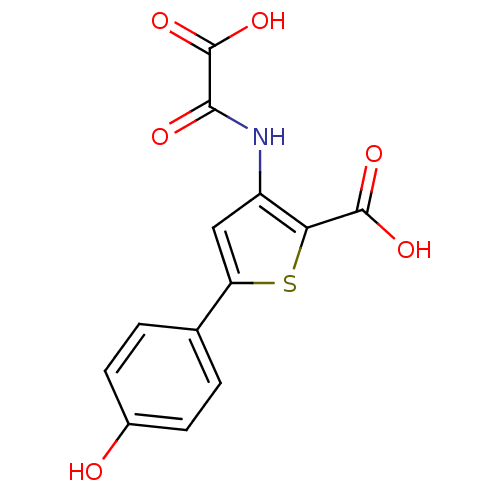 (3-(carboxyformamido)-5-(4-hydroxyphenyl)thiophene-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118780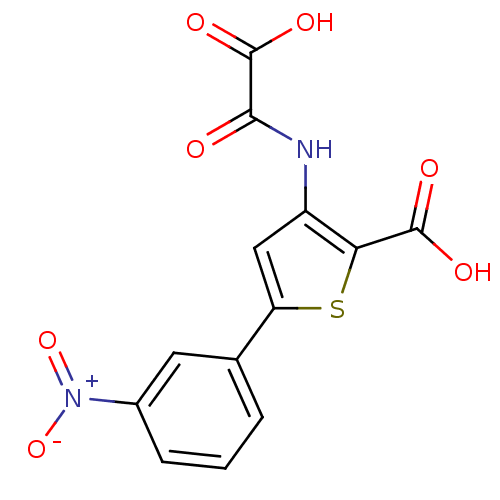 (3-(carboxyformamido)-5-(3-nitrophenyl)thiophene-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118783 (2-(Oxalyl-amino)-6-oxo-4,5,6,7-tetrahydro-6lambda*...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118758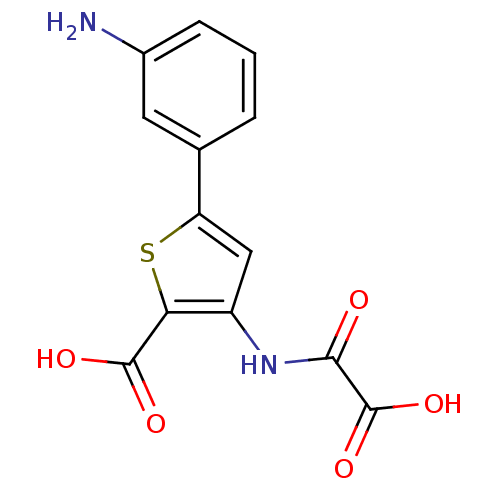 (5-(3-Amino-phenyl)-3-(oxalyl-amino)-thiophene-2-ca...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118763 (2-(Oxalyl-amino)-6,6-dioxo-4,5,6,7-tetrahydro-6lam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118740 (3-(carboxyformamido)-5-(4-fluorophenyl)thiophene-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118756 (3-(carboxyformamido)-5-(3,5-dimethoxyphenyl)thioph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118796 (6-(OXALYL-AMINO)-1H-INDOLE-5-CARBOXYLIC ACID | 6-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118762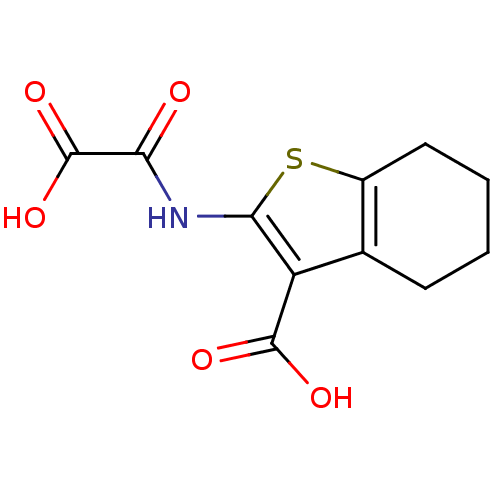 (2-(Oxalyl-amino)-4,5,6,7-tetrahydro-benzo[b]thioph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118787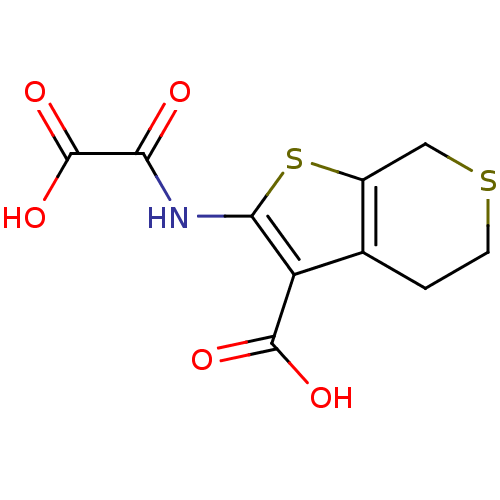 (2-(Oxalyl-amino)-4,7-dihydro-5H-thieno[2,3-c]thiop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118759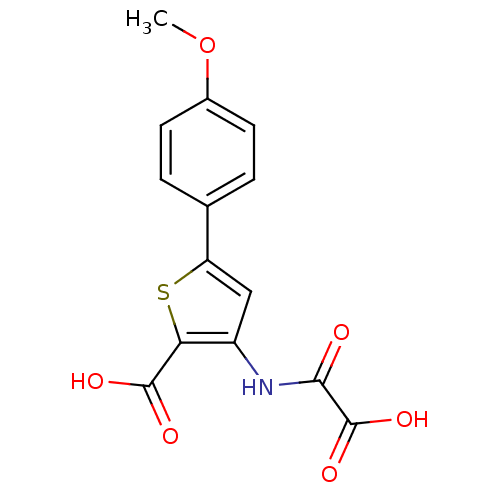 (3-(carboxyformamido)-5-(4-methoxyphenyl)thiophene-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118760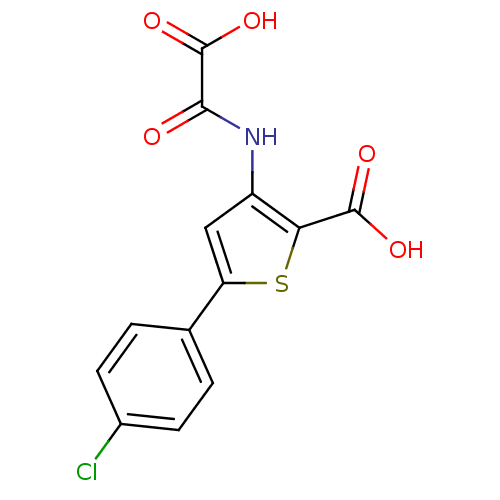 (3-(carboxyformamido)-5-(4-chlorophenyl)thiophene-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118764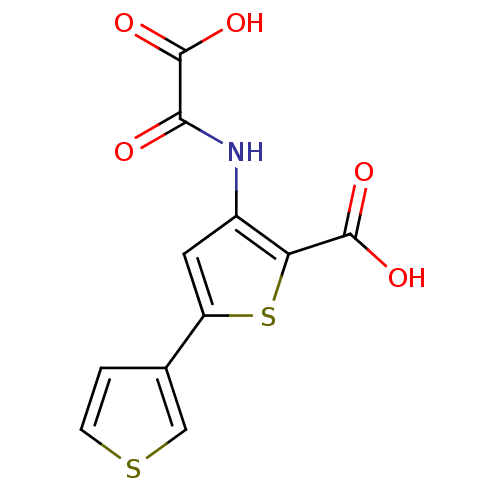 (3-(carboxyformamido)-5-(thiophen-3-yl)thiophene-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118788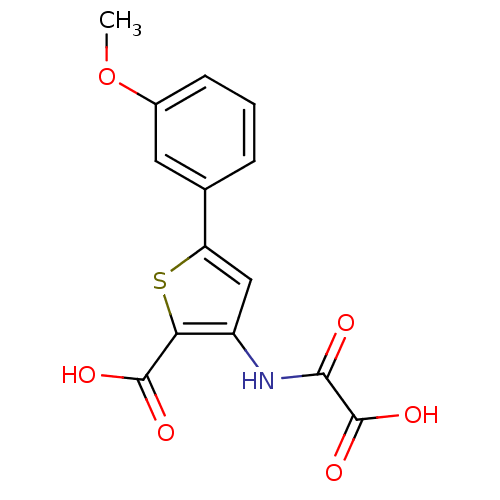 (3-(carboxyformamido)-5-(3-methoxyphenyl)thiophene-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118785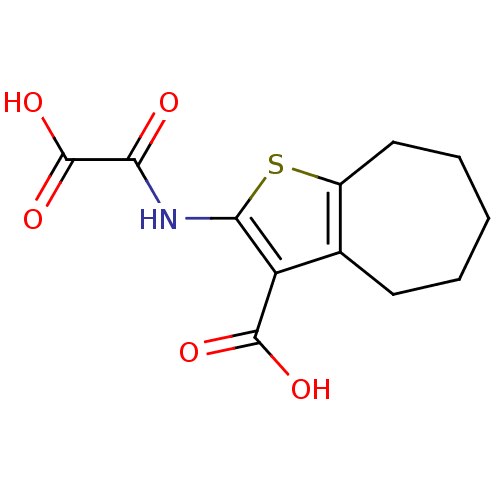 (2-(Oxalyl-amino)-5,6,7,8-tetrahydro-4H-cyclohepta[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118753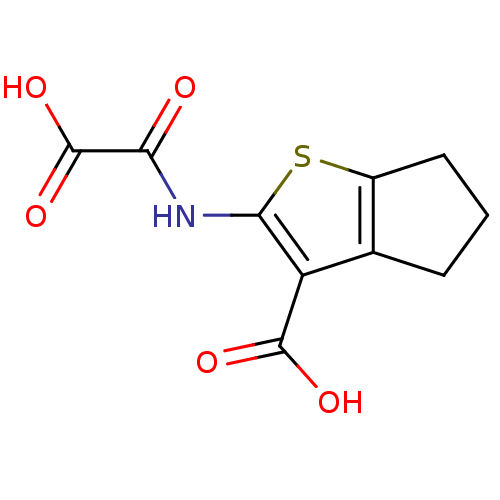 (2-(Oxalyl-amino)-5,6-dihydro-4H-cyclopenta[b]thiop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118747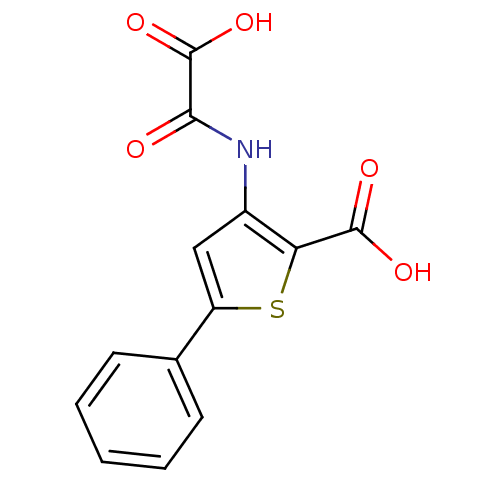 (3-(Oxalyl-amino)-5-phenyl-thiophene-2-carboxylic a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118781 (3-(carboxyformamido)-5-(4-isobutylphenyl)thiophene...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118776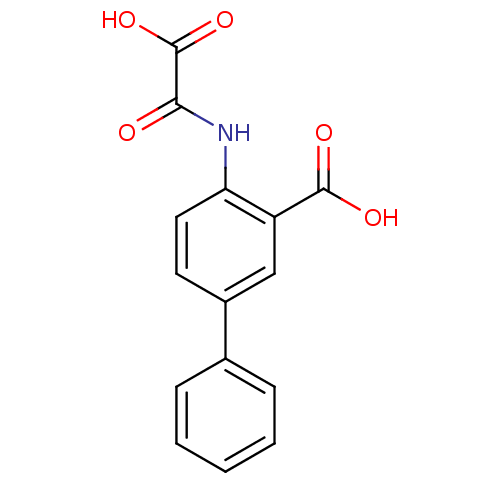 (4-(Oxalyl-amino)-biphenyl-3-carboxylic acid | 4-(c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118778 (5-iodo-2-(oxaloamino)benzoic acid | CHEMBL336908) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human PTP1B (Protein Tyrosine phosphatase 1B) using p-nitrophenyl phosphate substrate at pH 5.5 | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118744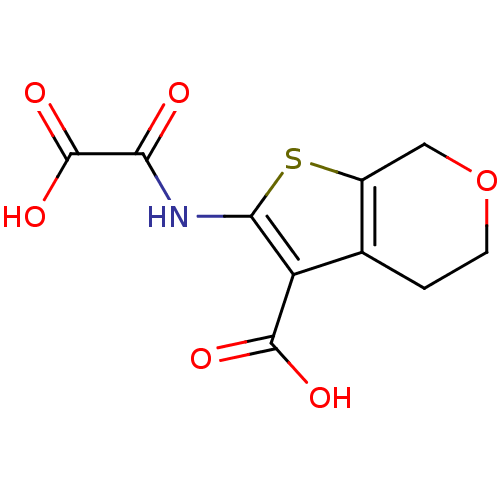 (2-(Oxalyl-amino)-4,7-dihydro-5H-thieno[2,3-c]pyran...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118745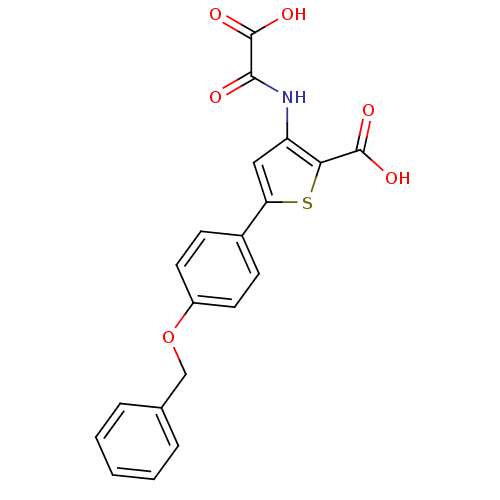 (5-(4-(benzyloxy)phenyl)-3-(carboxyformamido)thioph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118751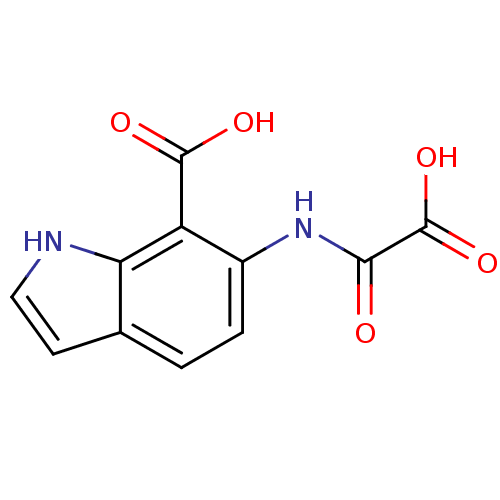 (6-(Oxalyl-amino)-1H-indole-7-carboxylic acid | 6-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118789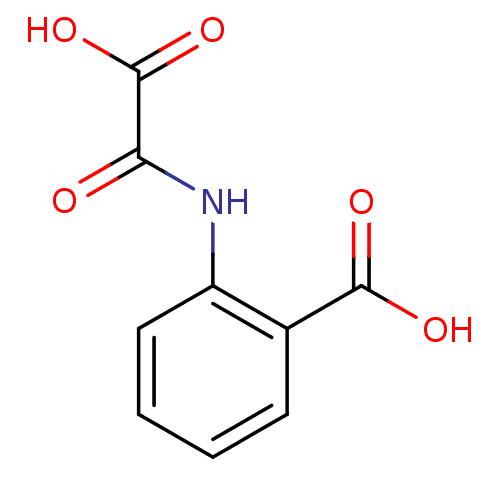 (2-(oxaloamino)benzoic acid | CHEMBL139050) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118769 (3-(carboxyformamido)-5-(3,4-dimethoxyphenyl)thioph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118775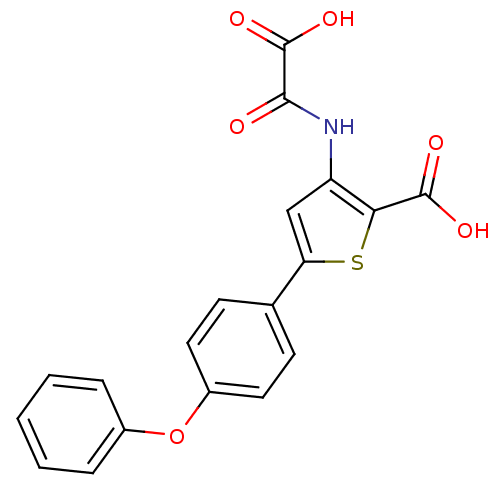 (3-(Oxalyl-amino)-5-(4-phenoxy-phenyl)-thiophene-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118779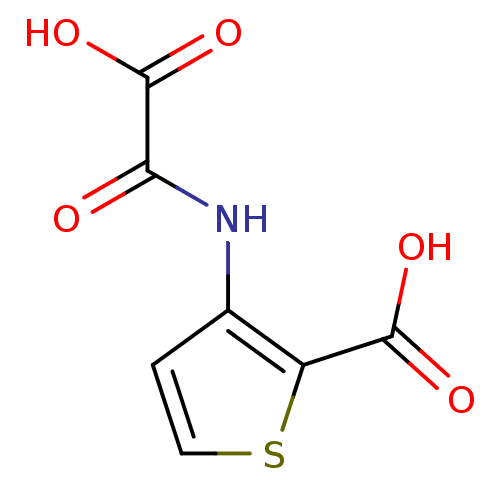 (3-(Oxalyl-amino)-thiophene-2-carboxylic acid | 3-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118795 (4-(Oxalyl-amino)-thiophene-3-carboxylic acid | 4-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human Protein-tyrosine phosphatase 1B (PTP1B) using p-nitrophenyl phosphate substrate at pH 5.5;ND is not deter... | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118749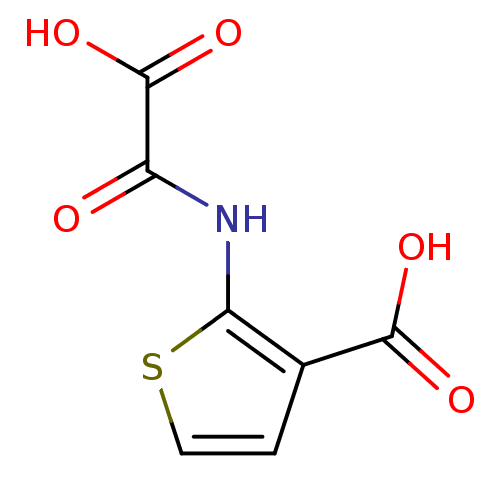 (2-(Oxalyl-amino)-thiophene-3-carboxylic acid | 2-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.08E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118746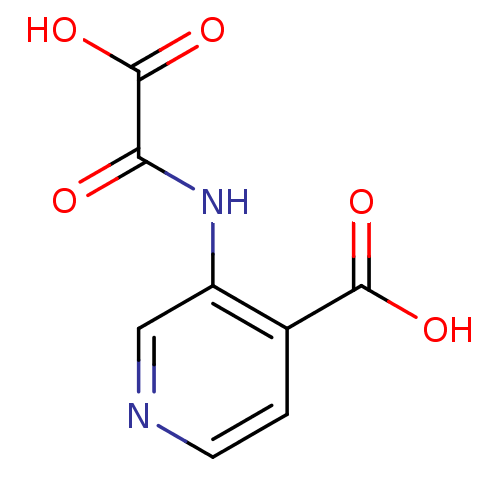 (3-(Oxalyl-amino)-isonicotinic acid | 3-(carboxyfor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118777 (2-(1-carboxy-N-methylformamido)benzoic acid | 2-(M...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118768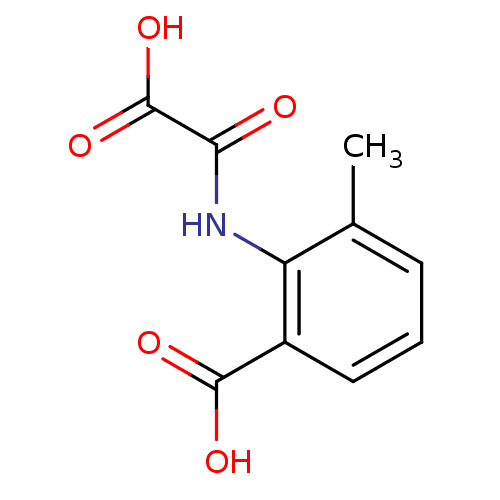 (2-(carboxyformamido)-3-methylbenzoic acid | 3-Meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human PTP1B (Protein Tyrosine phosphatase 1B) using p-nitrophenyl phosphate substrate at pH 5.5 | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118757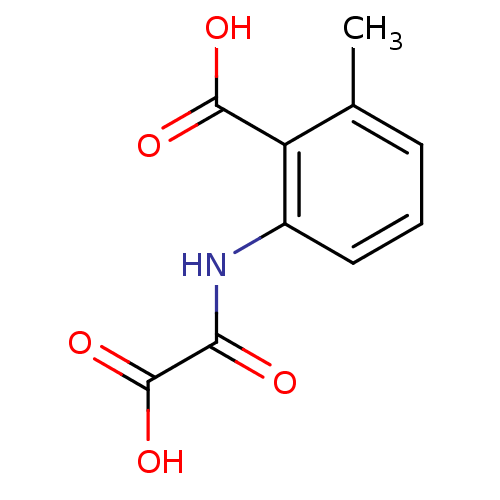 (2-(carboxyformamido)-6-methylbenzoic acid | 2-Meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50118743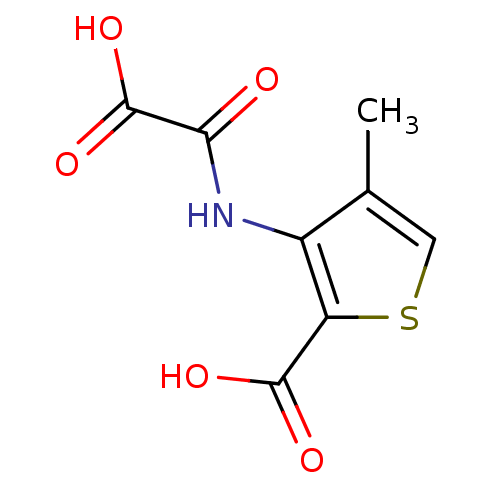 (3-(carboxyformamido)-4-methylthiophene-2-carboxyli...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human protein-tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate substrate at pH 5.5. | J Med Chem 45: 4443-59 (2002) BindingDB Entry DOI: 10.7270/Q2QJ7GM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM226157 (PTP1B spring 7 (7)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 3.00E+3 | -32.8 | 4.80E+3 | n/a | n/a | n/a | n/a | 6.0 | 37 | |
Catholic University of Daegu | Assay Description In each 96-well plates (total 200 μL of volume), there were 2 mM p-NPP and PTP1B (0.05-0.1 μg) in a buffer containing 50 mM citrate (pH 6.0... | Bioorg Chem 72: 273-281 (2017) BindingDB Entry DOI: 10.7270/Q2ZP450S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5625 total ) | Next | Last >> |