 Found 456 hits Enz. Inhib. hit(s) with Target = 'Serine/threonine-protein kinase MST2'
Found 456 hits Enz. Inhib. hit(s) with Target = 'Serine/threonine-protein kinase MST2' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM236557
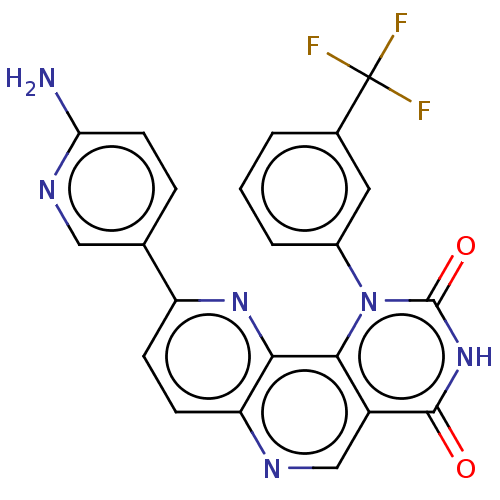
(US9365572, 5)Show SMILES Nc1ccc(cn1)-c1ccc2ncc3c(n(-c4cccc(c4)C(F)(F)F)c(=O)[nH]c3=O)c2n1 Show InChI InChI=1S/C22H13F3N6O2/c23-22(24,25)12-2-1-3-13(8-12)31-19-14(20(32)30-21(31)33)10-27-16-6-5-15(29-18(16)19)11-4-7-17(26)28-9-11/h1-10H,(H2,26,28)(H,30,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 28 |
Xuanzhu Pharma Co., Ltd.
US Patent
| Assay Description
Agents: 1-fold kinase buffer without MnCl2: 50 mM HEPES, pH 7.5, 0.0015% Brij-35, 10 mM MgCl2, 2 mM DTT. 1-fold kinase buffer with MnCl2: 50 mM HEPES... |
US Patent US9365572 (2016)
BindingDB Entry DOI: 10.7270/Q20C4TNC |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM25017
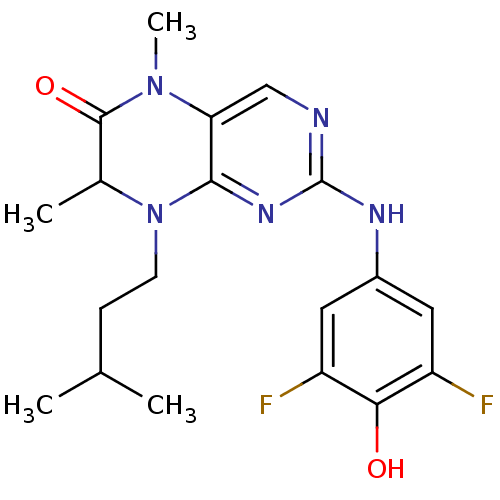
(2-[(3,5-difluoro-4-hydroxyphenyl)amino]-5,7-dimeth...)Show SMILES CC(C)CCN1C(C)C(=O)N(C)c2cnc(Nc3cc(F)c(O)c(F)c3)nc12 Show InChI InChI=1S/C19H23F2N5O2/c1-10(2)5-6-26-11(3)18(28)25(4)15-9-22-19(24-17(15)26)23-12-7-13(20)16(27)14(21)8-12/h7-11,27H,5-6H2,1-4H3,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 860 | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Dundee
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] labe... |
Biochem J 401: 29-38 (2007)
Article DOI: 10.1042/BJ20061088
BindingDB Entry DOI: 10.7270/Q25Q4TD7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50059345
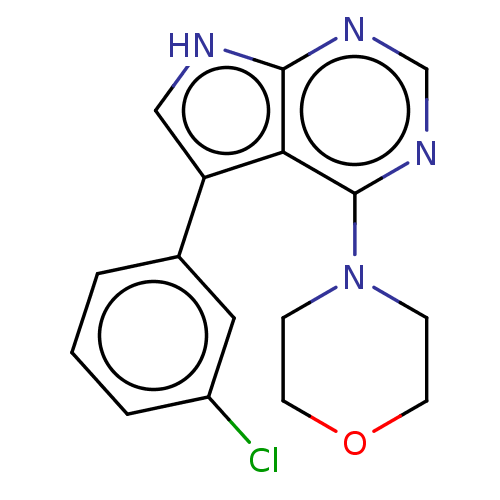
(CHEMBL3393355 | US9156845, 83)Show InChI InChI=1S/C16H15ClN4O/c17-12-3-1-2-11(8-12)13-9-18-15-14(13)16(20-10-19-15)21-4-6-22-7-5-21/h1-3,8-10H,4-7H2,(H,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant N-terminal His6-tagged human STK3 (18 to 311 residues) expressed in Escherichia coli assessed as inhibition constant |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00804
BindingDB Entry DOI: 10.7270/Q2Q52TH8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM185556
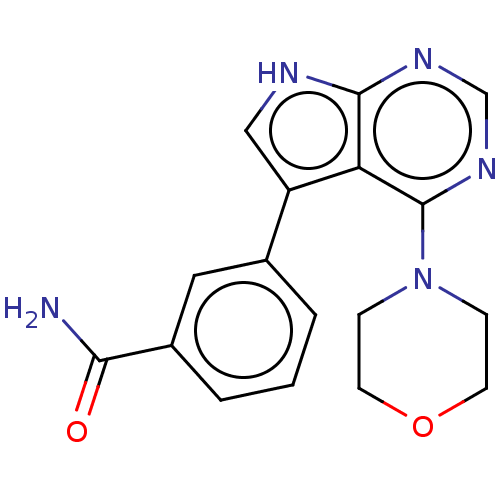
(US9156845, 215)Show SMILES NC(=O)c1cccc(c1)-c1c[nH]c2ncnc(N3CCOCC3)c12 Show InChI InChI=1S/C17H17N5O2/c18-15(23)12-3-1-2-11(8-12)13-9-19-16-14(13)17(21-10-20-16)22-4-6-24-7-5-22/h1-3,8-10H,4-7H2,(H2,18,23)(H,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant N-terminal His6-tagged human STK3 (18 to 311 residues) expressed in Escherichia coli assessed as inhibition constant |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00804
BindingDB Entry DOI: 10.7270/Q2Q52TH8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50583973
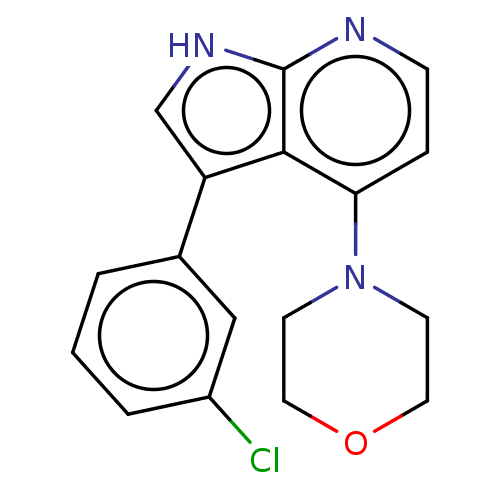
(CHEMBL5093114) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 211 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant N-terminal His6-tagged human STK3 (18 to 311 residues) expressed in Escherichia coli assessed as inhibition constant |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00804
BindingDB Entry DOI: 10.7270/Q2Q52TH8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50341519
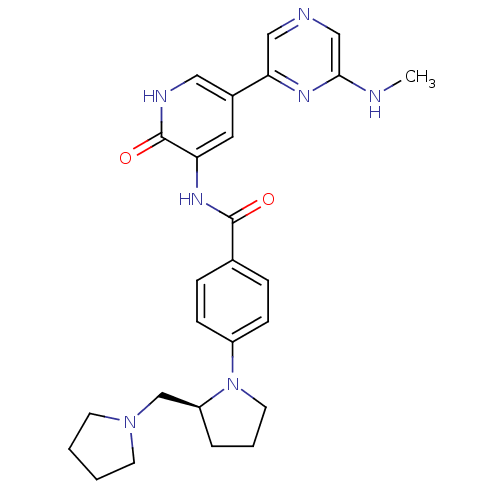
((S)-3-(4-(2-(pyrrolidin-1-ylmethyl)pyrrolidin-1-yl...)Show SMILES CNc1cncc(n1)-c1c[nH]c(=O)c(NC(=O)c2ccc(cc2)N2CCC[C@H]2CN2CCCC2)c1 |r| Show InChI InChI=1S/C26H31N7O2/c1-27-24-16-28-15-23(30-24)19-13-22(26(35)29-14-19)31-25(34)18-6-8-20(9-7-18)33-12-4-5-21(33)17-32-10-2-3-11-32/h6-9,13-16,21H,2-5,10-12,17H2,1H3,(H,27,30)(H,29,35)(H,31,34)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals (Europe) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of MST2 |
J Med Chem 54: 2341-50 (2011)
Article DOI: 10.1021/jm101499u
BindingDB Entry DOI: 10.7270/Q2KH0NPW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50402020
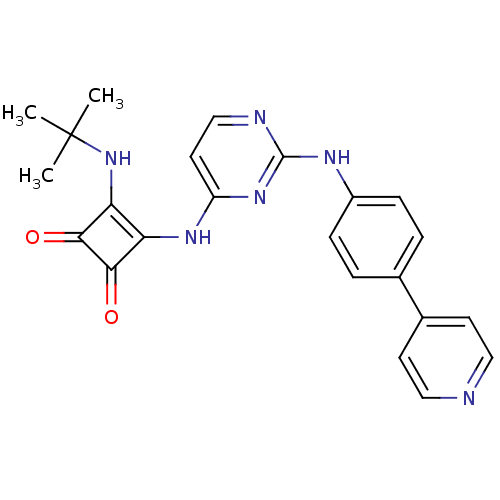
(CHEMBL2205426)Show SMILES CC(C)(C)Nc1c(Nc2ccnc(Nc3ccc(cc3)-c3ccncc3)n2)c(=O)c1=O Show InChI InChI=1S/C23H22N6O2/c1-23(2,3)29-19-18(20(30)21(19)31)27-17-10-13-25-22(28-17)26-16-6-4-14(5-7-16)15-8-11-24-12-9-15/h4-13,29H,1-3H3,(H2,25,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant MST2 after 1 hr by scintillation counter analysis in presence of gamma-[33P]ATP |
Bioorg Med Chem Lett 22: 7615-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.009
BindingDB Entry DOI: 10.7270/Q2XK8GQ3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50463484
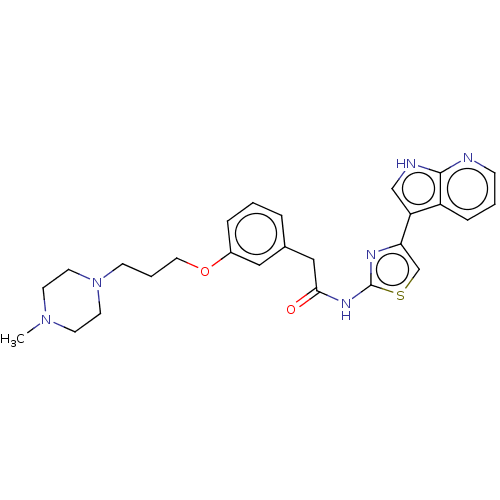
(CHEMBL4248525)Show SMILES CN1CCN(CCCOc2cccc(CC(=O)Nc3nc(cs3)-c3c[nH]c4ncccc34)c2)CC1 Show InChI InChI=1S/C26H30N6O2S/c1-31-10-12-32(13-11-31)9-4-14-34-20-6-2-5-19(15-20)16-24(33)30-26-29-23(18-35-26)22-17-28-25-21(22)7-3-8-27-25/h2-3,5-8,15,17-18H,4,9-14,16H2,1H3,(H,27,28)(H,29,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of MST2 (unknown origin) |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50463479

(CHEMBL4249925)Show SMILES CS(=O)(=O)Nc1cccc(CC(=O)Nc2nc(cs2)-c2c[nH]c3ncccc23)c1 Show InChI InChI=1S/C19H17N5O3S2/c1-29(26,27)24-13-5-2-4-12(8-13)9-17(25)23-19-22-16(11-28-19)15-10-21-18-14(15)6-3-7-20-18/h2-8,10-11,24H,9H2,1H3,(H,20,21)(H,22,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of MST2 (unknown origin) |
Bioorg Med Chem Lett 28: 2622-2626 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.040
BindingDB Entry DOI: 10.7270/Q2ZC85HX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM21079
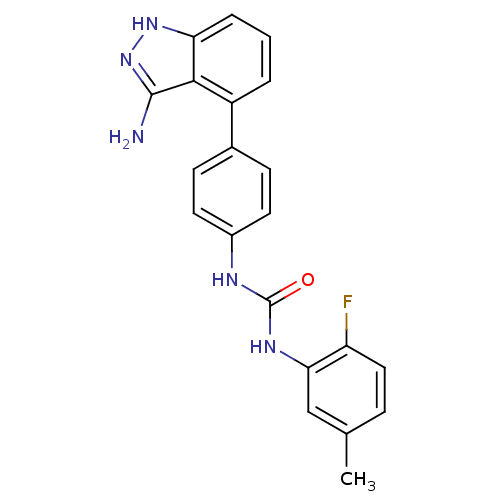
(1-[4-(3-amino-1H-indazol-4-yl)phenyl]-3-(2-fluoro-...)Show SMILES Cc1ccc(F)c(NC(=O)Nc2ccc(cc2)-c2cccc3[nH]nc(N)c23)c1 Show InChI InChI=1S/C21H18FN5O/c1-12-5-10-16(22)18(11-12)25-21(28)24-14-8-6-13(7-9-14)15-3-2-4-17-19(15)20(23)27-26-17/h2-11H,1H3,(H3,23,26,27)(H2,24,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2KH0KP2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
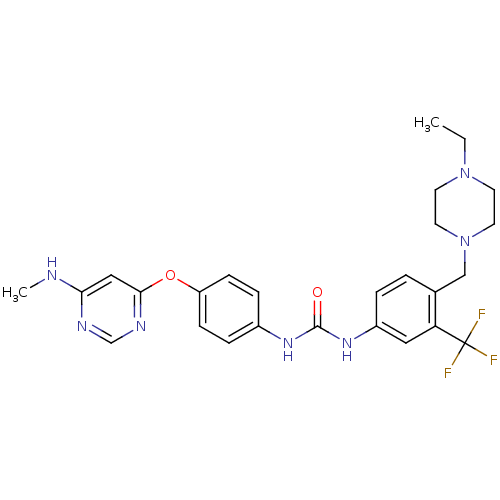
(Homo sapiens (Human)) | BDBM31085
(1-[4-[(4-ethyl-1-piperazinyl)methyl]-3-(trifluorom...)Show SMILES CCN1CCN(Cc2ccc(NC(=O)Nc3ccc(Oc4cc(NC)ncn4)cc3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H30F3N7O2/c1-3-35-10-12-36(13-11-35)16-18-4-5-20(14-22(18)26(27,28)29)34-25(37)33-19-6-8-21(9-7-19)38-24-15-23(30-2)31-17-32-24/h4-9,14-15,17H,3,10-13,16H2,1-2H3,(H,30,31,32)(H2,33,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PCBioAssay
| n/a | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2KH0KP2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
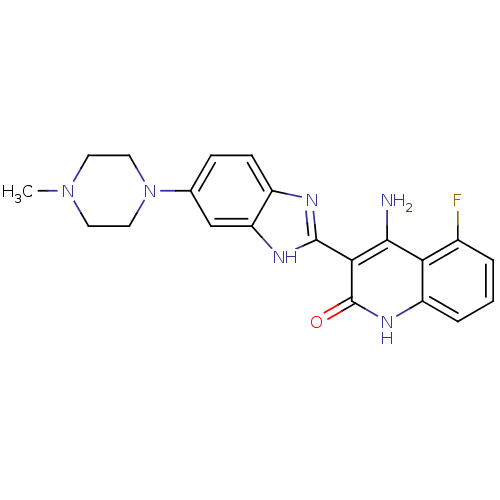
(Homo sapiens (Human)) | BDBM25118
((3Z)-4-amino-5-fluoro-3-[5-(4-methylpiperazino)-1,...)Show SMILES CN1CCN(CC1)c1ccc2nc([nH]c2c1)-c1c(N)c2c(F)cccc2[nH]c1=O Show InChI InChI=1S/C21H21FN6O/c1-27-7-9-28(10-8-27)12-5-6-14-16(11-12)25-20(24-14)18-19(23)17-13(22)3-2-4-15(17)26-21(18)29/h2-6,11H,7-10H2,1H3,(H,24,25)(H3,23,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2KH0KP2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2KH0KP2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
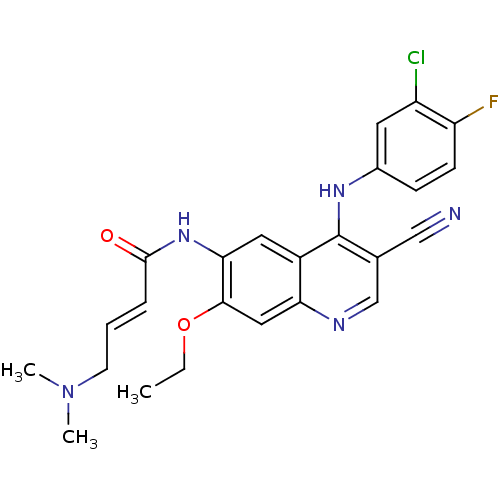
(Homo sapiens (Human)) | BDBM31090
((E)-N-[4-(3-chloro-4-fluoro-anilino)-3-cyano-7-eth...)Show SMILES CCOc1cc2ncc(C#N)c(Nc3ccc(F)c(Cl)c3)c2cc1NC(=O)\C=C\CN(C)C Show InChI InChI=1S/C24H23ClFN5O2/c1-4-33-22-12-20-17(11-21(22)30-23(32)6-5-9-31(2)3)24(15(13-27)14-28-20)29-16-7-8-19(26)18(25)10-16/h5-8,10-12,14H,4,9H2,1-3H3,(H,28,29)(H,30,32)/b6-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2KH0KP2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM31099
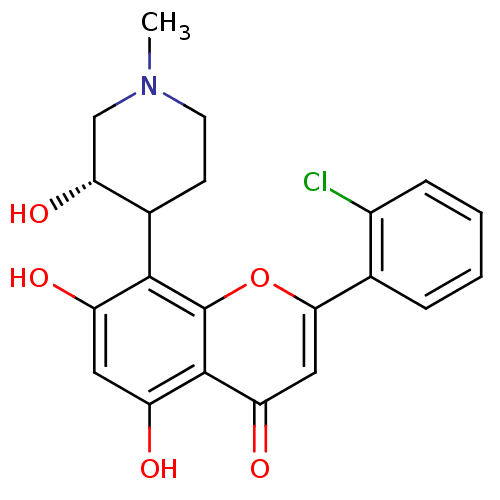
(2-(2-chlorophenyl)-5,7-dihydroxy-8-[(3S)-3-hydroxy...)Show SMILES CN1CCC([C@H](O)C1)c1c(O)cc(O)c2c1oc(cc2=O)-c1ccccc1Cl Show InChI InChI=1S/C21H20ClNO5/c1-23-7-6-12(17(27)10-23)19-14(24)8-15(25)20-16(26)9-18(28-21(19)20)11-4-2-3-5-13(11)22/h2-5,8-9,12,17,24-25,27H,6-7,10H2,1H3/t12?,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2KH0KP2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM6866
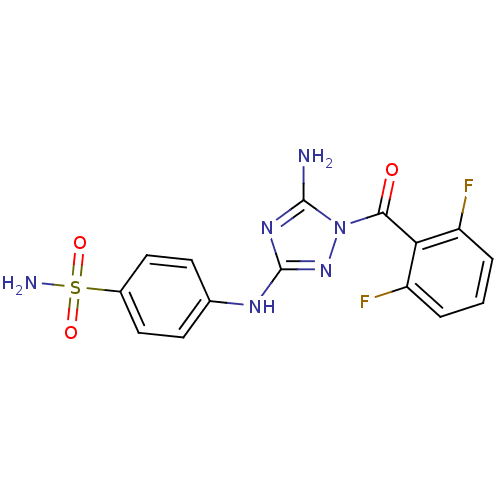
(1-Acyl-1H-[1,2,4]triazole-3,5-diamine Analogue 3b ...)Show SMILES Nc1nc(Nc2ccc(cc2)S(N)(=O)=O)nn1C(=O)c1c(F)cccc1F Show InChI InChI=1S/C15H12F2N6O3S/c16-10-2-1-3-11(17)12(10)13(24)23-14(18)21-15(22-23)20-8-4-6-9(7-5-8)27(19,25)26/h1-7H,(H2,19,25,26)(H3,18,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2KH0KP2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM17055
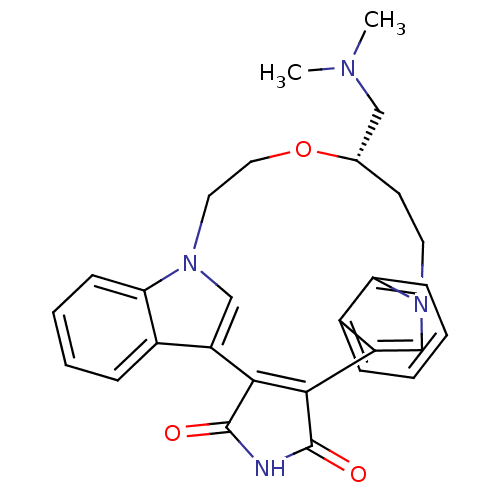
((18S)-18-[(dimethylamino)methyl]-17-oxa-4,14,21-tr...)Show SMILES CN(C)C[C@@H]1CCn2cc(C3=C(C(=O)NC3=O)c3cn(CCO1)c1ccccc31)c1ccccc21 |r,t:10| Show InChI InChI=1S/C28H28N4O3/c1-30(2)15-18-11-12-31-16-21(19-7-3-5-9-23(19)31)25-26(28(34)29-27(25)33)22-17-32(13-14-35-18)24-10-6-4-8-20(22)24/h3-10,16-18H,11-15H2,1-2H3,(H,29,33,34)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2KH0KP2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM31094
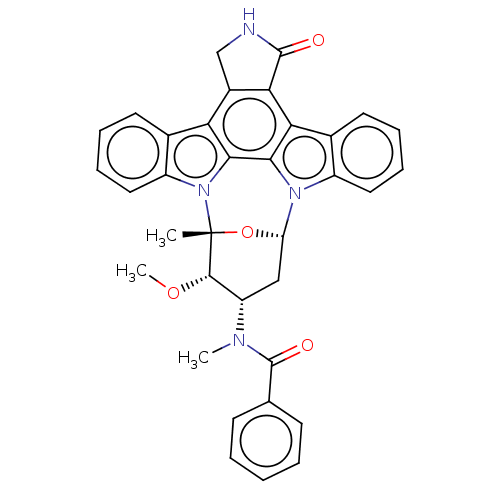
(PKC-412 | cid_24202429)Show SMILES [H][C@@]12C[C@@H]([C@H](OC)[C@@](C)(O1)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |TLB:11:10:9:4.3.2,17:33:9:4.3.2,THB:24:32:9:4.3.2,5:4:9:10.31.33.32,30:31:9:4.3.2| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2KH0KP2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM31095
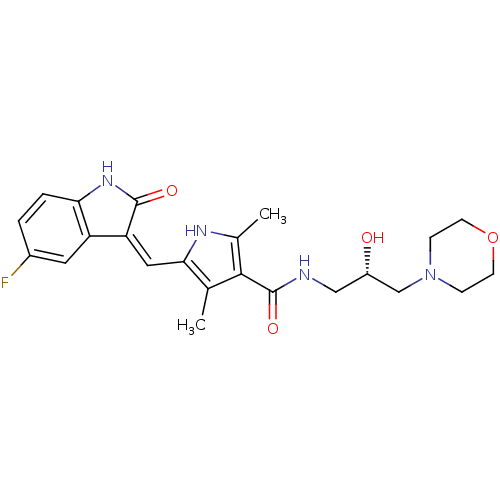
(5-[(Z)-(5-fluoranyl-2-oxidanylidene-1H-indol-3-yli...)Show SMILES Cc1[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c(C)c1C(=O)NC[C@H](O)CN1CCOCC1 Show InChI InChI=1S/C23H27FN4O4/c1-13-20(10-18-17-9-15(24)3-4-19(17)27-22(18)30)26-14(2)21(13)23(31)25-11-16(29)12-28-5-7-32-8-6-28/h3-4,9-10,16,26,29H,5-8,11-12H2,1-2H3,(H,25,31)(H,27,30)/b18-10-/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2KH0KP2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
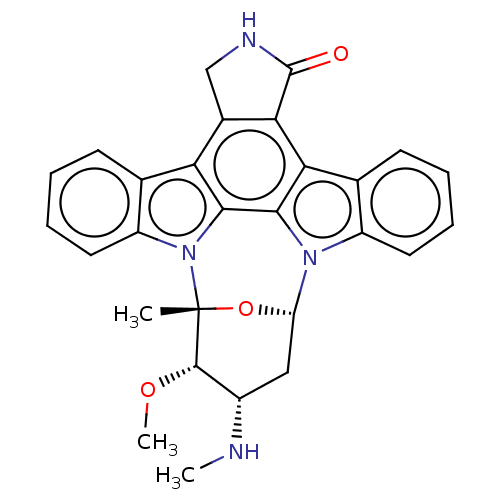
(Homo sapiens (Human)) | BDBM31096
(CHEMBL290084 | Staurosporine | cid_451705)Show SMILES CN[C@H]1C[C@@H]2O[C@](C)([C@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2KH0KP2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
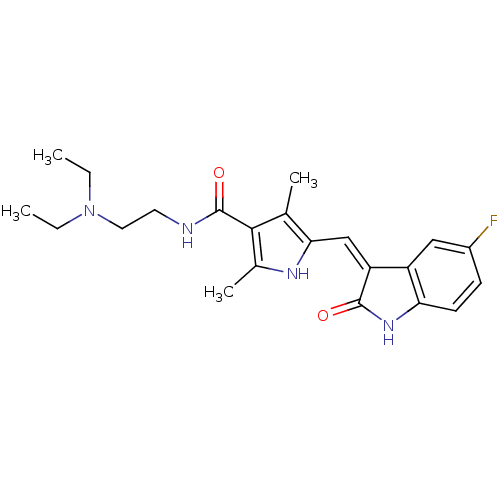
(Homo sapiens (Human)) | BDBM4814
(CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2KH0KP2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PCBioAssay
| n/a | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by PubChem BioAssay
| Assay Description
Kinase inhibitors are a new class of therapeutics with a propensity to inhibit multiple targets. The biological consequences of multi-kinase activity... |
PubChem Bioassay (2008)
BindingDB Entry DOI: 10.7270/Q2KH0KP2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
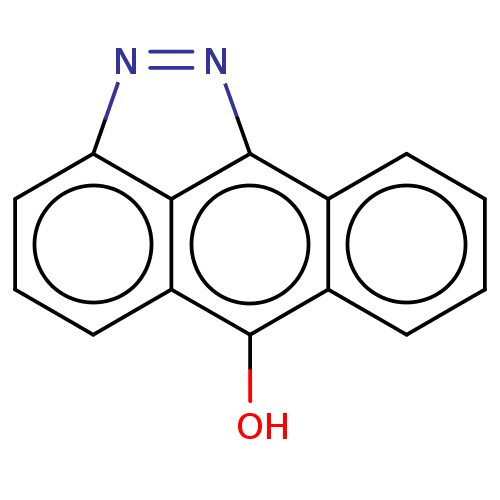
(Mus musculus) | BDBM50024294
(SP-600125)Show InChI InChI=1S/C14H8N2O/c17-14-9-5-2-1-4-8(9)13-12-10(14)6-3-7-11(12)15-16-13/h1-7,17H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Average Binding Constant for STK3_m; NA=Not Active at 10 uM |
Nat Biotechnol 23: 329-36 (2005)
Article DOI: 10.1038/nbt1068
BindingDB Entry DOI: 10.7270/Q2V69J3T |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Mus musculus) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Average Binding Constant for STK3_m; NA=Not Active at 10 uM |
Nat Biotechnol 23: 329-36 (2005)
Article DOI: 10.1038/nbt1068
BindingDB Entry DOI: 10.7270/Q2V69J3T |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50326053

(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to MST2 |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50308060
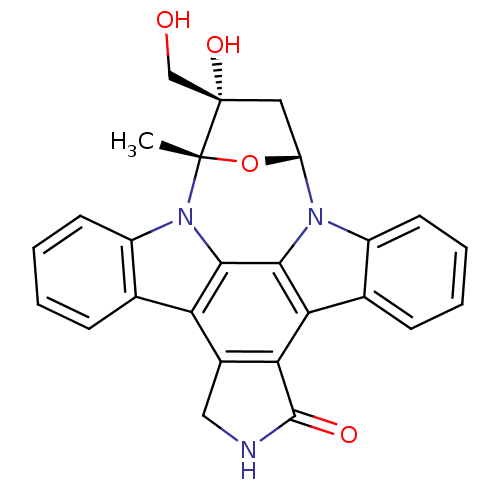
(16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...)Show SMILES C[C@]12O[C@H](C[C@]1(O)CO)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding affinity to MST2 |
Blood 114: 2984-92 (2009)
Article DOI: 10.1182/blood-2009-05-222034
BindingDB Entry DOI: 10.7270/Q2PN95V2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50026612
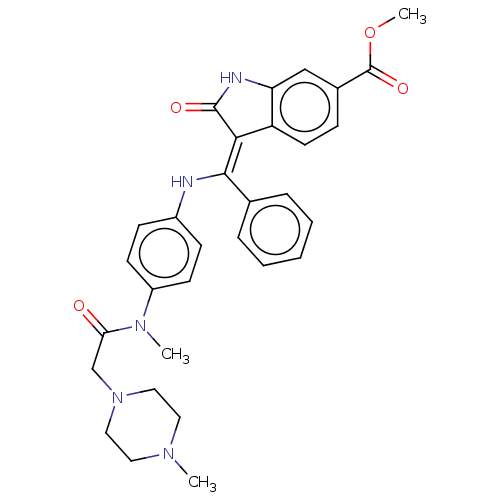
(BIBF-1120 | Nintedanib | US10981896, Compound Nint...)Show SMILES COC(=O)c1ccc2\C(=C(\Nc3ccc(cc3)N(C)C(=O)CN3CCN(C)CC3)c3ccccc3)C(=O)Nc2c1 Show InChI InChI=1S/C31H33N5O4/c1-34-15-17-36(18-16-34)20-27(37)35(2)24-12-10-23(11-13-24)32-29(21-7-5-4-6-8-21)28-25-14-9-22(31(39)40-3)19-26(25)33-30(28)38/h4-14,19,32H,15-18,20H2,1-3H3,(H,33,38)/b29-28- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for MST2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for MST2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50308060

(16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...)Show SMILES C[C@]12O[C@H](C[C@]1(O)CO)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for MST2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50326053

(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for MST2 kinase domain |
Nat Biotechnol 29: 1046-51 (2011)
Article DOI: 10.1038/nbt.1990
BindingDB Entry DOI: 10.7270/Q25D8S70 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50335188
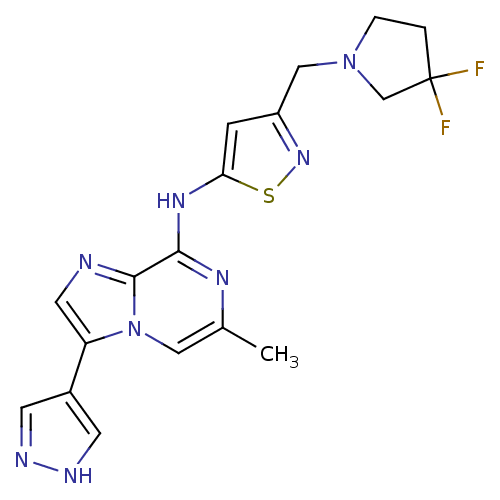
(CHEMBL1650545 | CHEMBL1650551 | N-[3-[(3,3-Difluor...)Show SMILES Cc1cn2c(cnc2c(Nc2cc(CN3CCC(F)(F)C3)ns2)n1)-c1cn[nH]c1 Show InChI InChI=1S/C18H18F2N8S/c1-11-8-28-14(12-5-22-23-6-12)7-21-17(28)16(24-11)25-15-4-13(26-29-15)9-27-3-2-18(19,20)10-27/h4-8H,2-3,9-10H2,1H3,(H,22,23)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MST2 |
J Med Chem 54: 201-10 (2011)
Article DOI: 10.1021/jm1010995
BindingDB Entry DOI: 10.7270/Q2VM4D76 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of MST2 |
J Med Chem 52: 3191-204 (2009)
Article DOI: 10.1021/jm800861c
BindingDB Entry DOI: 10.7270/Q23J3DWT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for MST2 kinase domain |
Nat Biotechnol 26: 127-32 (2008)
Article DOI: 10.1038/nbt1358
BindingDB Entry DOI: 10.7270/Q2TT4RX2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50326053

(CHEMBL608533 | PKC-412)Show SMILES CO[C@@H]1[C@@H](C[C@H]2O[C@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13)N(C)C(=O)c1ccccc1 |r| Show InChI InChI=1S/C35H30N4O4/c1-35-32(42-3)25(37(2)34(41)19-11-5-4-6-12-19)17-26(43-35)38-23-15-9-7-13-20(23)28-29-22(18-36-33(29)40)27-21-14-8-10-16-24(21)39(35)31(27)30(28)38/h4-16,25-26,32H,17-18H2,1-3H3,(H,36,40)/t25-,26-,32-,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Binding constant for MST2 kinase domain |
Nat Biotechnol 26: 127-32 (2008)
Article DOI: 10.1038/nbt1358
BindingDB Entry DOI: 10.7270/Q2TT4RX2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50030881
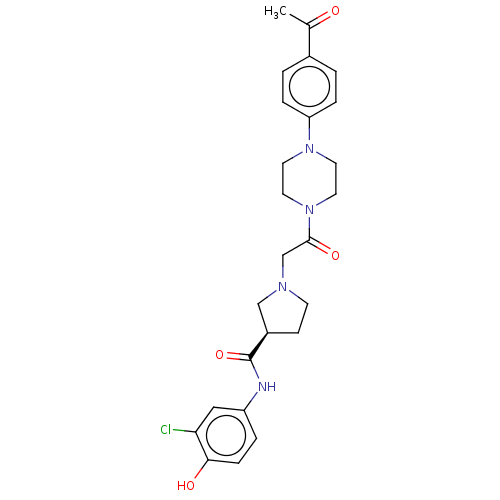
(CHEMBL3356117)Show SMILES CC(=O)c1ccc(cc1)N1CCN(CC1)C(=O)CN1CC[C@H](C1)C(=O)Nc1ccc(O)c(Cl)c1 |r| Show InChI InChI=1S/C25H29ClN4O4/c1-17(31)18-2-5-21(6-3-18)29-10-12-30(13-11-29)24(33)16-28-9-8-19(15-28)25(34)27-20-4-7-23(32)22(26)14-20/h2-7,14,19,32H,8-13,15-16H2,1H3,(H,27,34)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MST2 (unknown origin) |
J Med Chem 57: 8817-26 (2014)
Article DOI: 10.1021/jm500847m
BindingDB Entry DOI: 10.7270/Q2Q52R6D |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50045370
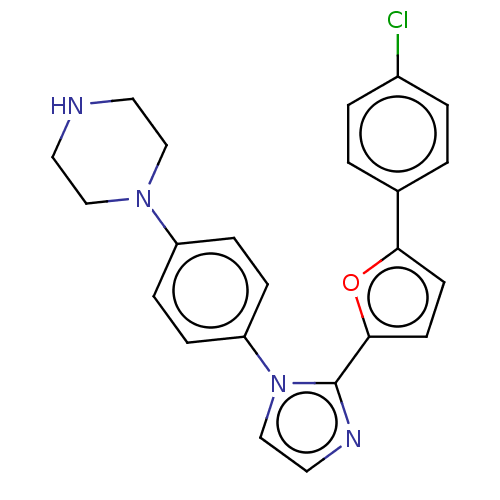
(CHEMBL3314140)Show SMILES Clc1ccc(cc1)-c1ccc(o1)-c1nccn1-c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C23H21ClN4O/c24-18-3-1-17(2-4-18)21-9-10-22(29-21)23-26-13-16-28(23)20-7-5-19(6-8-20)27-14-11-25-12-15-27/h1-10,13,16,25H,11-12,14-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MST2 (unknown origin) |
Bioorg Med Chem Lett 24: 3609-13 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.024
BindingDB Entry DOI: 10.7270/Q2F47QR7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50043416
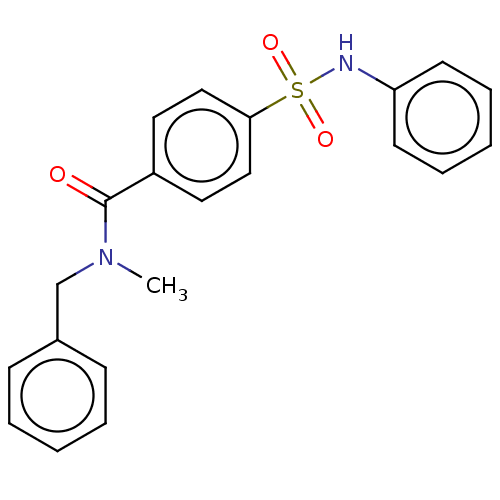
(CHEMBL3355482)Show SMILES CN(Cc1ccccc1)C(=O)c1ccc(cc1)S(=O)(=O)Nc1ccccc1 Show InChI InChI=1S/C21H20N2O3S/c1-23(16-17-8-4-2-5-9-17)21(24)18-12-14-20(15-13-18)27(25,26)22-19-10-6-3-7-11-19/h2-15,22H,16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lexicon Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of MST2 (unknown origin) in presence of 1 mM ATP |
ACS Med Chem Lett 6: 53-7 (2015)
Article DOI: 10.1021/ml500242y
BindingDB Entry DOI: 10.7270/Q2SQ9207 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50059277
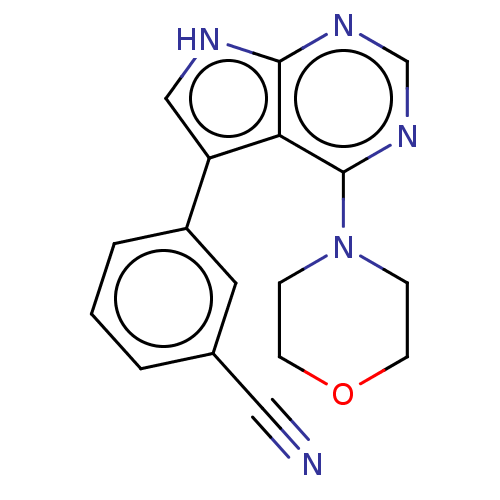
(CHEMBL3393348 | US9156845, 4)Show InChI InChI=1S/C17H15N5O/c18-9-12-2-1-3-13(8-12)14-10-19-16-15(14)17(21-11-20-16)22-4-6-23-7-5-22/h1-3,8,10-11H,4-7H2,(H,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MST2 using Ser/Thr peptide 7 substrate after 45 mins by Z-Lyte assay |
J Med Chem 58: 419-32 (2015)
Article DOI: 10.1021/jm5014055
BindingDB Entry DOI: 10.7270/Q2XS5X2W |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50059281
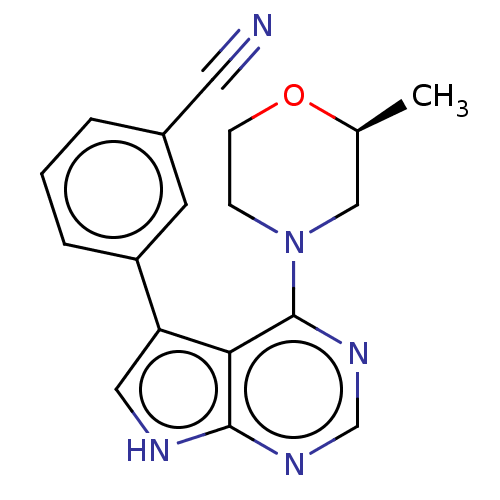
(CHEMBL3393444 | US9156845, 6)Show SMILES C[C@H]1CN(CCO1)c1ncnc2[nH]cc(-c3cccc(c3)C#N)c12 |r| Show InChI InChI=1S/C18H17N5O/c1-12-10-23(5-6-24-12)18-16-15(9-20-17(16)21-11-22-18)14-4-2-3-13(7-14)8-19/h2-4,7,9,11-12H,5-6,10H2,1H3,(H,20,21,22)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 179 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MST2 using Ser/Thr peptide 7 substrate after 45 mins by Z-Lyte assay |
J Med Chem 58: 419-32 (2015)
Article DOI: 10.1021/jm5014055
BindingDB Entry DOI: 10.7270/Q2XS5X2W |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50059284
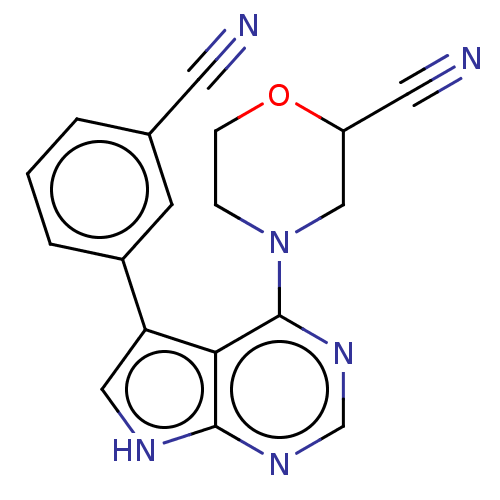
(CHEMBL3393445)Show SMILES N#CC1CN(CCO1)c1ncnc2[nH]cc(-c3cccc(c3)C#N)c12 Show InChI InChI=1S/C18H14N6O/c19-7-12-2-1-3-13(6-12)15-9-21-17-16(15)18(23-11-22-17)24-4-5-25-14(8-20)10-24/h1-3,6,9,11,14H,4-5,10H2,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 288 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MST2 using Ser/Thr peptide 7 substrate after 45 mins by Z-Lyte assay |
J Med Chem 58: 419-32 (2015)
Article DOI: 10.1021/jm5014055
BindingDB Entry DOI: 10.7270/Q2XS5X2W |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50059309
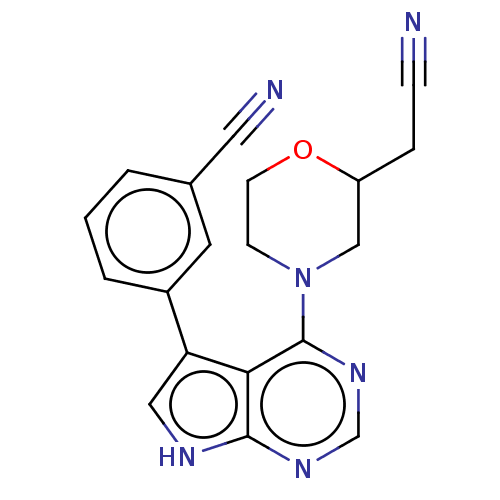
(CHEMBL3393446)Show SMILES N#CCC1CN(CCO1)c1ncnc2[nH]cc(-c3cccc(c3)C#N)c12 Show InChI InChI=1S/C19H16N6O/c20-5-4-15-11-25(6-7-26-15)19-17-16(10-22-18(17)23-12-24-19)14-3-1-2-13(8-14)9-21/h1-3,8,10,12,15H,4,6-7,11H2,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 196 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MST2 using Ser/Thr peptide 7 substrate after 45 mins by Z-Lyte assay |
J Med Chem 58: 419-32 (2015)
Article DOI: 10.1021/jm5014055
BindingDB Entry DOI: 10.7270/Q2XS5X2W |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50059310
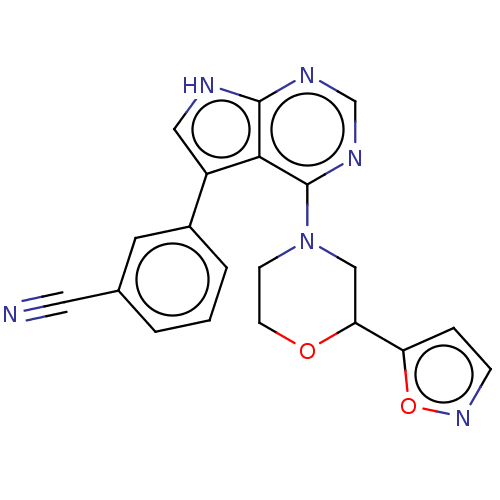
(CHEMBL3393447 | US9156845, 219)Show SMILES N#Cc1cccc(c1)-c1c[nH]c2ncnc(N3CCOC(C3)c3ccno3)c12 Show InChI InChI=1S/C20H16N6O2/c21-9-13-2-1-3-14(8-13)15-10-22-19-18(15)20(24-12-23-19)26-6-7-27-17(11-26)16-4-5-25-28-16/h1-5,8,10,12,17H,6-7,11H2,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MST2 using Ser/Thr peptide 7 substrate after 45 mins by Z-Lyte assay |
J Med Chem 58: 419-32 (2015)
Article DOI: 10.1021/jm5014055
BindingDB Entry DOI: 10.7270/Q2XS5X2W |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50059290
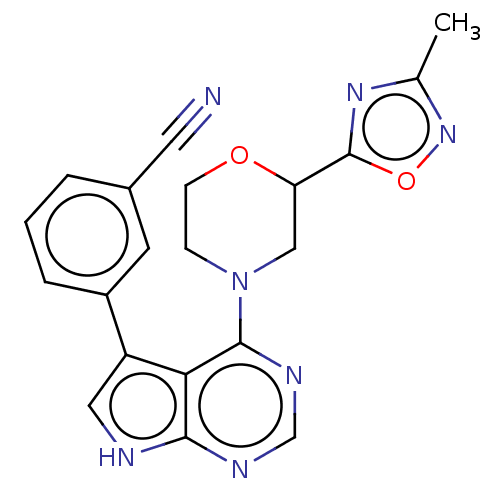
(CHEMBL3393448 | US9156845, 110)Show SMILES Cc1noc(n1)C1CN(CCO1)c1ncnc2[nH]cc(-c3cccc(c3)C#N)c12 Show InChI InChI=1S/C20H17N7O2/c1-12-25-20(29-26-12)16-10-27(5-6-28-16)19-17-15(9-22-18(17)23-11-24-19)14-4-2-3-13(7-14)8-21/h2-4,7,9,11,16H,5-6,10H2,1H3,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MST2 using Ser/Thr peptide 7 substrate after 45 mins by Z-Lyte assay |
J Med Chem 58: 419-32 (2015)
Article DOI: 10.1021/jm5014055
BindingDB Entry DOI: 10.7270/Q2XS5X2W |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50059294
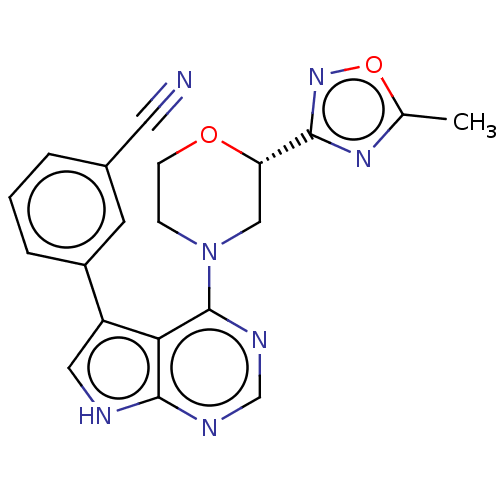
(CHEMBL3393449)Show SMILES Cc1nc(no1)[C@@H]1CN(CCO1)c1ncnc2[nH]cc(-c3cccc(c3)C#N)c12 |r| Show InChI InChI=1S/C20H17N7O2/c1-12-25-18(26-29-12)16-10-27(5-6-28-16)20-17-15(9-22-19(17)23-11-24-20)14-4-2-3-13(7-14)8-21/h2-4,7,9,11,16H,5-6,10H2,1H3,(H,22,23,24)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 795 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MST2 using Ser/Thr peptide 7 substrate after 45 mins by Z-Lyte assay |
J Med Chem 58: 419-32 (2015)
Article DOI: 10.1021/jm5014055
BindingDB Entry DOI: 10.7270/Q2XS5X2W |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50059295
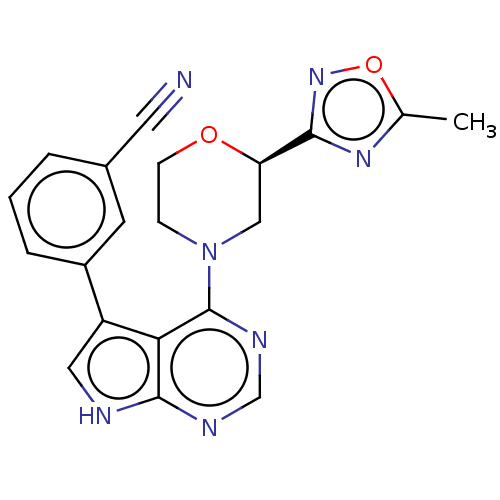
(CHEMBL3393450)Show SMILES Cc1nc(no1)[C@H]1CN(CCO1)c1ncnc2[nH]cc(-c3cccc(c3)C#N)c12 |r| Show InChI InChI=1S/C20H17N7O2/c1-12-25-18(26-29-12)16-10-27(5-6-28-16)20-17-15(9-22-19(17)23-11-24-20)14-4-2-3-13(7-14)8-21/h2-4,7,9,11,16H,5-6,10H2,1H3,(H,22,23,24)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MST2 using Ser/Thr peptide 7 substrate after 45 mins by Z-Lyte assay |
J Med Chem 58: 419-32 (2015)
Article DOI: 10.1021/jm5014055
BindingDB Entry DOI: 10.7270/Q2XS5X2W |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50059282
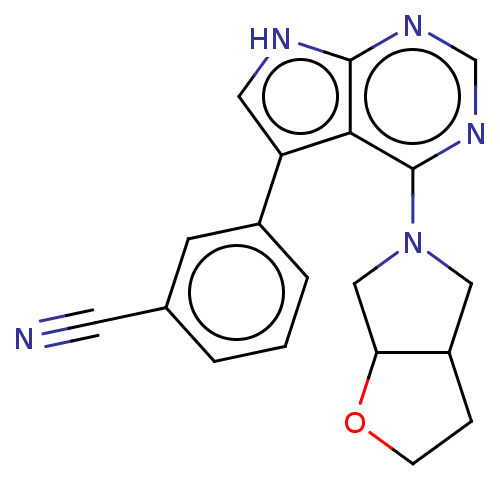
(CHEMBL3393453)Show SMILES N#Cc1cccc(c1)-c1c[nH]c2ncnc(N3CC4CCOC4C3)c12 Show InChI InChI=1S/C19H17N5O/c20-7-12-2-1-3-13(6-12)15-8-21-18-17(15)19(23-11-22-18)24-9-14-4-5-25-16(14)10-24/h1-3,6,8,11,14,16H,4-5,9-10H2,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 626 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MST2 using Ser/Thr peptide 7 substrate after 45 mins by Z-Lyte assay |
J Med Chem 58: 419-32 (2015)
Article DOI: 10.1021/jm5014055
BindingDB Entry DOI: 10.7270/Q2XS5X2W |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Mus musculus) | BDBM4814

(CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Average Binding Constant for STK3_m; NA=Not Active at 10 uM |
Nat Biotechnol 23: 329-36 (2005)
Article DOI: 10.1038/nbt1068
BindingDB Entry DOI: 10.7270/Q2V69J3T |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Mus musculus) | BDBM17055

((18S)-18-[(dimethylamino)methyl]-17-oxa-4,14,21-tr...)Show SMILES CN(C)C[C@@H]1CCn2cc(C3=C(C(=O)NC3=O)c3cn(CCO1)c1ccccc31)c1ccccc21 |r,t:10| Show InChI InChI=1S/C28H28N4O3/c1-30(2)15-18-11-12-31-16-21(19-7-3-5-9-23(19)31)25-26(28(34)29-27(25)33)22-17-32(13-14-35-18)24-10-6-4-8-20(22)24/h3-10,16-18H,11-15H2,1-2H3,(H,29,33,34)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a |
Ambit Biosciences
Curated by ChEMBL
| Assay Description
Average Binding Constant for STK3_m; NA=Not Active at 10 uM |
Nat Biotechnol 23: 329-36 (2005)
Article DOI: 10.1038/nbt1068
BindingDB Entry DOI: 10.7270/Q2V69J3T |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50273541
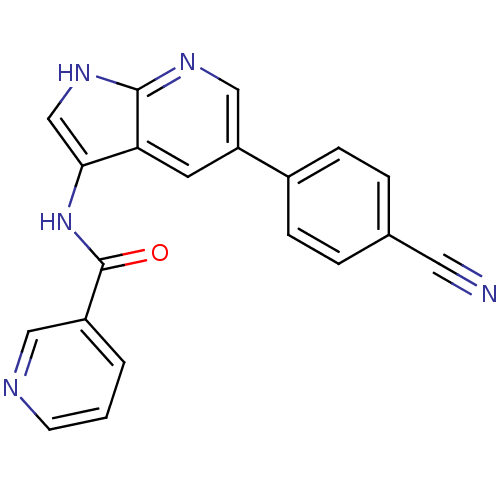
(CHEMBL516312 | N-(5-(4-cyanophenyl)-1H-pyrrolo[2,3...)Show SMILES O=C(Nc1c[nH]c2ncc(cc12)-c1ccc(cc1)C#N)c1cccnc1 Show InChI InChI=1S/C20H13N5O/c21-9-13-3-5-14(6-4-13)16-8-17-18(12-24-19(17)23-11-16)25-20(26)15-2-1-7-22-10-15/h1-8,10-12H,(H,23,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to human MST2 |
J Med Chem 51: 7898-914 (2008)
Article DOI: 10.1021/jm8011036
BindingDB Entry DOI: 10.7270/Q2WS8T4C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50193995

(3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyr...)Show SMILES C[C@@H]1CCN(C[C@@H]1N(C)c1ncnc2[nH]ccc12)C(=O)CC#N |r| Show InChI InChI=1S/C16H20N6O/c1-11-5-8-22(14(23)3-6-17)9-13(11)21(2)16-12-4-7-18-15(12)19-10-20-16/h4,7,10-11,13H,3,5,8-9H2,1-2H3,(H,18,19,20)/t11-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to MST2 |
J Med Chem 51: 8012-8 (2008)
Article DOI: 10.1021/jm801142b
BindingDB Entry DOI: 10.7270/Q29W0FB7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data