

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM112686 (US8629282, 57) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Membranes were prepared from CHO cells expressing human S1P1. Cells were dissociated in buffer containing 20 mM HEPES, pH 7.5, 50 mM NaCl, 2 mM EDTA ... | US Patent US8629282 (2014) BindingDB Entry DOI: 10.7270/Q28914JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM112688 (US8629282, 68) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Membranes were prepared from CHO cells expressing human S1P1. Cells were dissociated in buffer containing 20 mM HEPES, pH 7.5, 50 mM NaCl, 2 mM EDTA ... | US Patent US8629282 (2014) BindingDB Entry DOI: 10.7270/Q28914JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM112687 (US8629282, 65) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Membranes were prepared from CHO cells expressing human S1P1. Cells were dissociated in buffer containing 20 mM HEPES, pH 7.5, 50 mM NaCl, 2 mM EDTA ... | US Patent US8629282 (2014) BindingDB Entry DOI: 10.7270/Q28914JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM112677 (US8629282, 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Membranes were prepared from CHO cells expressing human S1P1. Cells were dissociated in buffer containing 20 mM HEPES, pH 7.5, 50 mM NaCl, 2 mM EDTA ... | US Patent US8629282 (2014) BindingDB Entry DOI: 10.7270/Q28914JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM112681 (US8629282, 30) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Membranes were prepared from CHO cells expressing human S1P1. Cells were dissociated in buffer containing 20 mM HEPES, pH 7.5, 50 mM NaCl, 2 mM EDTA ... | US Patent US8629282 (2014) BindingDB Entry DOI: 10.7270/Q28914JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM112685 (US8629282, 53) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Membranes were prepared from CHO cells expressing human S1P1. Cells were dissociated in buffer containing 20 mM HEPES, pH 7.5, 50 mM NaCl, 2 mM EDTA ... | US Patent US8629282 (2014) BindingDB Entry DOI: 10.7270/Q28914JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM112683 (US8629282, 41) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Membranes were prepared from CHO cells expressing human S1P1. Cells were dissociated in buffer containing 20 mM HEPES, pH 7.5, 50 mM NaCl, 2 mM EDTA ... | US Patent US8629282 (2014) BindingDB Entry DOI: 10.7270/Q28914JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM112676 (US8629282, 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Membranes were prepared from CHO cells expressing human S1P1. Cells were dissociated in buffer containing 20 mM HEPES, pH 7.5, 50 mM NaCl, 2 mM EDTA ... | US Patent US8629282 (2014) BindingDB Entry DOI: 10.7270/Q28914JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM112680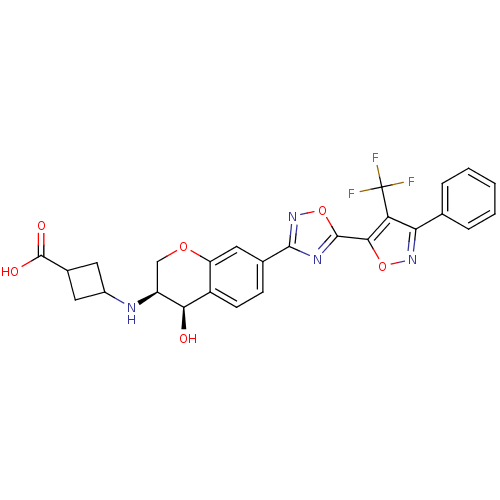 (US8629282, 25) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Membranes were prepared from CHO cells expressing human S1P1. Cells were dissociated in buffer containing 20 mM HEPES, pH 7.5, 50 mM NaCl, 2 mM EDTA ... | US Patent US8629282 (2014) BindingDB Entry DOI: 10.7270/Q28914JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM112678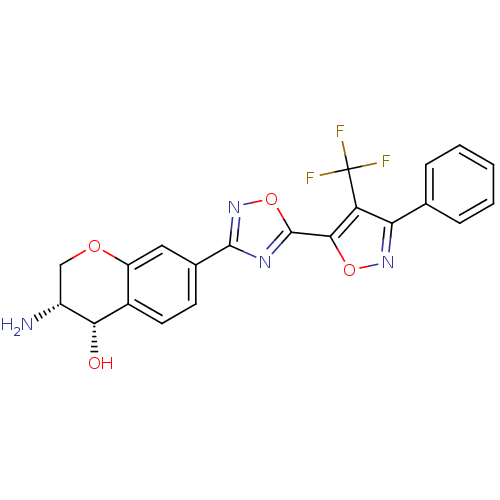 (US8629282, 9) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 39 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Membranes were prepared from CHO cells expressing human S1P1. Cells were dissociated in buffer containing 20 mM HEPES, pH 7.5, 50 mM NaCl, 2 mM EDTA ... | US Patent US8629282 (2014) BindingDB Entry DOI: 10.7270/Q28914JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM112684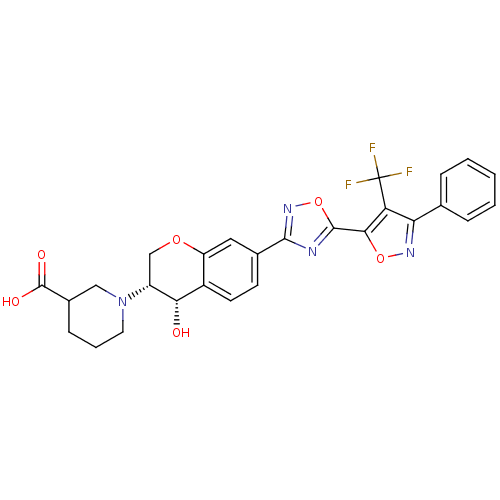 (US8629282, 50) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 57 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Membranes were prepared from CHO cells expressing human S1P1. Cells were dissociated in buffer containing 20 mM HEPES, pH 7.5, 50 mM NaCl, 2 mM EDTA ... | US Patent US8629282 (2014) BindingDB Entry DOI: 10.7270/Q28914JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM112682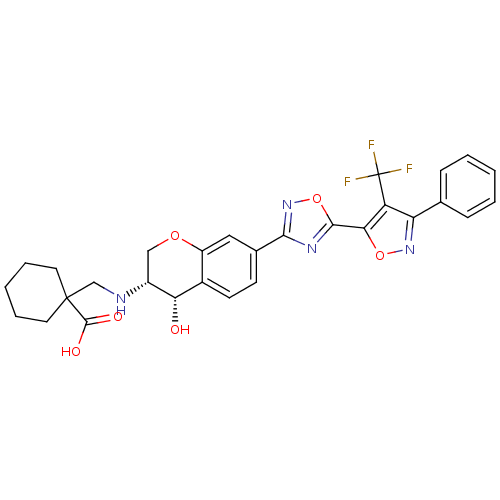 (US8629282, 39) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 541 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Membranes were prepared from CHO cells expressing human S1P1. Cells were dissociated in buffer containing 20 mM HEPES, pH 7.5, 50 mM NaCl, 2 mM EDTA ... | US Patent US8629282 (2014) BindingDB Entry DOI: 10.7270/Q28914JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM112679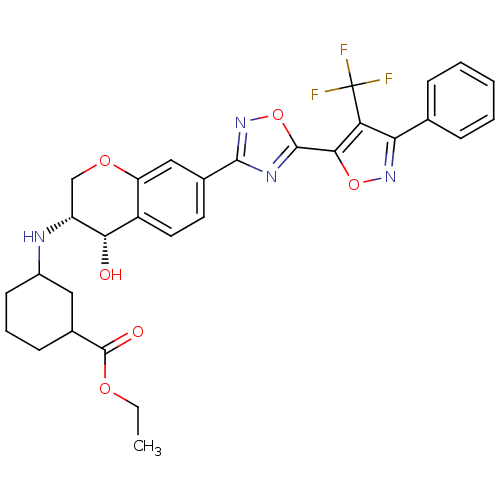 (US8629282, 13) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 630 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Membranes were prepared from CHO cells expressing human S1P1. Cells were dissociated in buffer containing 20 mM HEPES, pH 7.5, 50 mM NaCl, 2 mM EDTA ... | US Patent US8629282 (2014) BindingDB Entry DOI: 10.7270/Q28914JD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||