

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM120265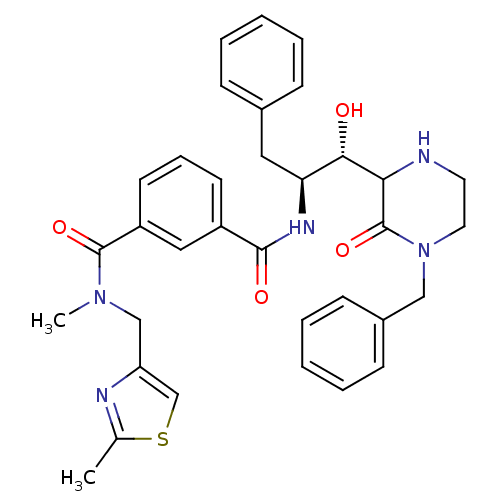 (US8703947, 11a | US8703947, 11b) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1.00E+3 | <-35.6 | <1.00E+4 | n/a | n/a | n/a | n/a | 4.0 | 37 |
Purdue Research Foundation US Patent | Assay Description The potencies of compounds were determined by measurement of their inhibition of memapsin 2 catalytic activity toward a fluorescent substrate. Kineti... | US Patent US8703947 (2014) BindingDB Entry DOI: 10.7270/Q2154FQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM120275 (US8703947, GRL- 1069AL) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <1.00E+3 | <-35.6 | n/a | n/a | n/a | n/a | n/a | 4.0 | 37 |
Purdue Research Foundation US Patent | Assay Description The potencies of compounds were determined by measurement of their inhibition of memapsin 2 catalytic activity toward a fluorescent substrate. Kineti... | US Patent US8703947 (2014) BindingDB Entry DOI: 10.7270/Q2154FQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM120270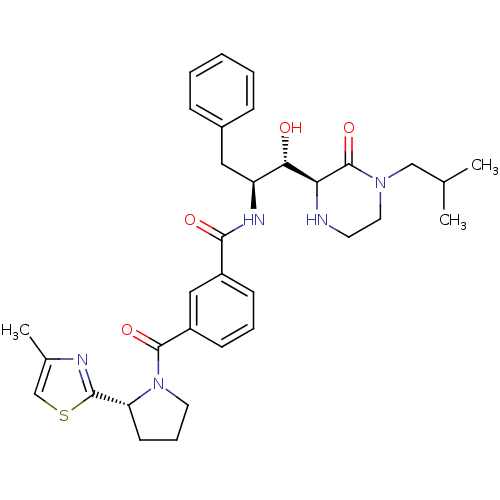 (US8703947, GRL- 0669AL) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <1.00E+3 | <-35.6 | n/a | n/a | n/a | n/a | n/a | 4.0 | 37 |
Purdue Research Foundation US Patent | Assay Description The potencies of compounds were determined by measurement of their inhibition of memapsin 2 catalytic activity toward a fluorescent substrate. Kineti... | US Patent US8703947 (2014) BindingDB Entry DOI: 10.7270/Q2154FQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM120273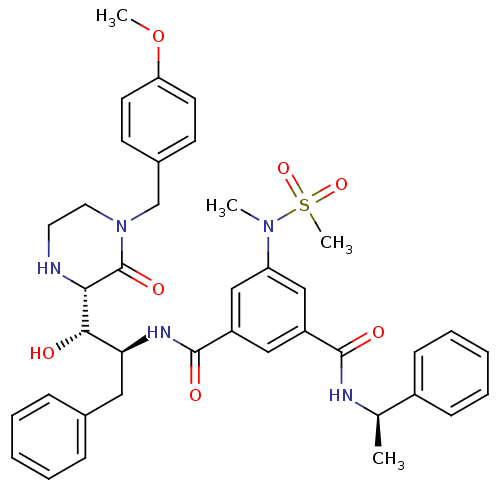 (US8703947, GRL- 0889AL) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <1.00E+3 | <-35.6 | n/a | n/a | n/a | n/a | n/a | 4.0 | 37 |
Purdue Research Foundation US Patent | Assay Description The potencies of compounds were determined by measurement of their inhibition of memapsin 2 catalytic activity toward a fluorescent substrate. Kineti... | US Patent US8703947 (2014) BindingDB Entry DOI: 10.7270/Q2154FQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM120276 (US8703947, GRL- 1079AL) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <1.00E+3 | <-35.6 | n/a | n/a | n/a | n/a | n/a | 4.0 | 37 |
Purdue Research Foundation US Patent | Assay Description The potencies of compounds were determined by measurement of their inhibition of memapsin 2 catalytic activity toward a fluorescent substrate. Kineti... | US Patent US8703947 (2014) BindingDB Entry DOI: 10.7270/Q2154FQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM120268 (US8703947, GRL- 0299) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <1.00E+3 | <-35.6 | n/a | n/a | n/a | n/a | n/a | 4.0 | 37 |
Purdue Research Foundation US Patent | Assay Description The potencies of compounds were determined by measurement of their inhibition of memapsin 2 catalytic activity toward a fluorescent substrate. Kineti... | US Patent US8703947 (2014) BindingDB Entry DOI: 10.7270/Q2154FQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM120271 (US8703947, GRL- 0819AL) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <1.00E+3 | <-35.6 | n/a | n/a | n/a | n/a | n/a | 4.0 | 37 |
Purdue Research Foundation US Patent | Assay Description The potencies of compounds were determined by measurement of their inhibition of memapsin 2 catalytic activity toward a fluorescent substrate. Kineti... | US Patent US8703947 (2014) BindingDB Entry DOI: 10.7270/Q2154FQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM120272 (US8703947, GRL- 0849AL) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <1.00E+3 | <-35.6 | n/a | n/a | n/a | n/a | n/a | 4.0 | 37 |
Purdue Research Foundation US Patent | Assay Description The potencies of compounds were determined by measurement of their inhibition of memapsin 2 catalytic activity toward a fluorescent substrate. Kineti... | US Patent US8703947 (2014) BindingDB Entry DOI: 10.7270/Q2154FQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM120265 (US8703947, 11a | US8703947, 11b) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >1.00E+4 | >-29.7 | n/a | n/a | n/a | n/a | n/a | 4.0 | 37 |
Purdue Research Foundation US Patent | Assay Description The potencies of compounds were determined by measurement of their inhibition of memapsin 2 catalytic activity toward a fluorescent substrate. Kineti... | US Patent US8703947 (2014) BindingDB Entry DOI: 10.7270/Q2154FQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM120264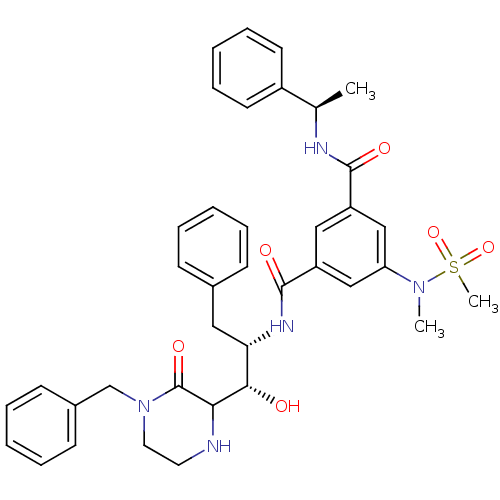 (US8703947, 10) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <1.00E+4 | <-29.7 | n/a | n/a | n/a | n/a | n/a | 4.0 | 37 |
Purdue Research Foundation US Patent | Assay Description The potencies of compounds were determined by measurement of their inhibition of memapsin 2 catalytic activity toward a fluorescent substrate. Kineti... | US Patent US8703947 (2014) BindingDB Entry DOI: 10.7270/Q2154FQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM120267 (US8703947, Comparison compound B) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >1.00E+4 | >-29.7 | n/a | n/a | n/a | n/a | n/a | 4.0 | 37 |
Purdue Research Foundation US Patent | Assay Description The potencies of compounds were determined by measurement of their inhibition of memapsin 2 catalytic activity toward a fluorescent substrate. Kineti... | US Patent US8703947 (2014) BindingDB Entry DOI: 10.7270/Q2154FQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||