 Found 231 hits of Enzyme Inhibition Constant Data
Found 231 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121503

(US8722683, 50)Show SMILES O=C(N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1)C1COc2ccccc2C1 |r,wU:3.2,wD:6.6,(4.23,-.82,;5,.52,;4.23,1.85,;2.69,1.85,;1.92,.52,;.38,.52,;-.38,1.85,;-1.92,1.85,;-2.69,.52,;-4.23,.52,;-5,-.82,;-6.54,-.82,;-7.31,.52,;-6.54,1.85,;-5,1.85,;-8.85,.52,;-9.62,1.85,;-11.16,1.85,;-11.93,.52,;-11.16,-.82,;-11.64,-2.28,;-10.39,-3.19,;-9.15,-2.28,;-9.62,-.82,;.38,3.19,;1.92,3.19,;6.54,.52,;7.31,-.82,;8.85,-.82,;9.62,.52,;11.16,.52,;11.93,1.85,;11.16,3.19,;9.62,3.19,;8.85,1.85,;7.31,1.85,)| Show InChI InChI=1S/C29H37N3O4/c33-29(23-18-22-4-1-2-6-26(22)34-19-23)30-24-10-8-21(9-11-24)12-13-31-14-16-32(17-15-31)25-5-3-7-27-28(25)36-20-35-27/h1-7,21,23-24H,8-20H2,(H,30,33)/t21-,23?,24- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121467

(US8722683, 14)Show SMILES O=C(CC#N)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:6.5,wD:9.9,(6.16,-.82,;6.93,.52,;8.47,.52,;9.24,-.82,;10.01,-2.15,;6.16,1.85,;4.62,1.85,;3.85,.52,;2.31,.52,;1.54,1.85,;,1.85,;-.77,.52,;-2.31,.52,;-3.08,-.82,;-4.62,-.82,;-5.39,.52,;-4.62,1.85,;-3.08,1.85,;-6.93,.52,;-7.7,1.85,;-9.24,1.85,;-10.01,.52,;-9.24,-.82,;-9.72,-2.28,;-8.47,-3.19,;-7.22,-2.28,;-7.7,-.82,;2.31,3.19,;3.85,3.19,)| Show InChI InChI=1S/C22H30N4O3/c23-10-8-21(27)24-18-6-4-17(5-7-18)9-11-25-12-14-26(15-13-25)19-2-1-3-20-22(19)29-16-28-20/h1-3,17-18H,4-9,11-16H2,(H,24,27)/t17-,18- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121528

(US8722683, 75)Show SMILES Clc1ccc(NC(=O)N[C@H]2CC[C@H](CCN3CCN(CC3)c3cccc4OCOc34)CC2)cc1 |r,wU:9.8,wD:12.12,(10.78,4.52,;10.01,3.19,;8.47,3.19,;7.7,1.85,;8.47,.52,;7.7,-.82,;6.16,-.82,;5.39,-2.15,;5.39,.52,;3.85,.52,;3.08,-.82,;1.54,-.82,;.77,.52,;-.77,.52,;-1.54,-.82,;-3.08,-.82,;-3.85,-2.15,;-5.39,-2.15,;-6.16,-.82,;-5.39,.52,;-3.85,.52,;-7.7,-.82,;-8.47,.52,;-10.01,.52,;-10.78,-.82,;-10.01,-2.15,;-10.49,-3.61,;-9.24,-4.52,;-7.99,-3.61,;-8.47,-2.15,;1.54,1.85,;3.08,1.85,;10.01,.52,;10.78,1.85,)| Show InChI InChI=1S/C26H33ClN4O3/c27-20-6-10-22(11-7-20)29-26(32)28-21-8-4-19(5-9-21)12-13-30-14-16-31(17-15-30)23-2-1-3-24-25(23)34-18-33-24/h1-3,6-7,10-11,19,21H,4-5,8-9,12-18H2,(H2,28,29,32)/t19-,21- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121454

(US8722683, 1)Show SMILES CC(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:4.3,wD:7.7,(9.24,.52,;7.7,.52,;6.93,-.82,;6.93,1.85,;5.39,1.85,;4.62,.52,;3.08,.52,;2.31,1.85,;.77,1.85,;,.52,;-1.54,.52,;-2.31,-.82,;-3.85,-.82,;-4.62,.52,;-3.85,1.85,;-2.31,1.85,;-6.16,.52,;-6.93,1.85,;-8.47,1.85,;-9.24,.52,;-8.47,-.82,;-8.95,-2.28,;-7.7,-3.19,;-6.45,-2.28,;-6.93,-.82,;3.08,3.19,;4.62,3.19,)| Show InChI InChI=1S/C21H31N3O3/c1-16(25)22-18-7-5-17(6-8-18)9-10-23-11-13-24(14-12-23)19-3-2-4-20-21(19)27-15-26-20/h2-4,17-18H,5-15H2,1H3,(H,22,25)/t17-,18- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121461
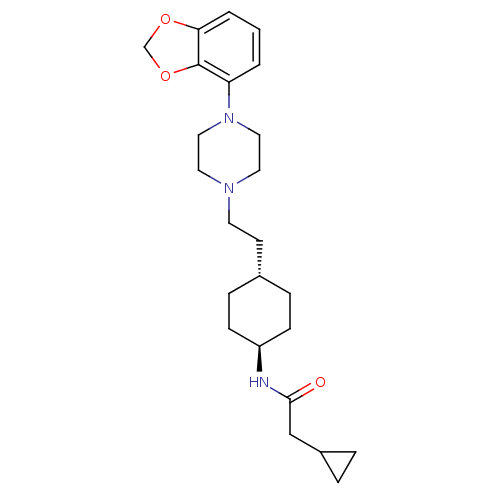
(US8722683, 8)Show SMILES O=C(CC1CC1)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:7.7,wD:10.11,(5.88,-.82,;6.65,.52,;8.19,.52,;8.96,-.82,;8.96,-2.36,;10.29,-1.59,;5.88,1.85,;4.34,1.85,;3.57,.52,;2.03,.52,;1.26,1.85,;-.28,1.85,;-1.05,.52,;-2.59,.52,;-3.36,-.82,;-4.9,-.82,;-5.67,.52,;-4.9,1.85,;-3.36,1.85,;-7.21,.52,;-7.98,1.85,;-9.52,1.85,;-10.29,.52,;-9.52,-.82,;-10,-2.28,;-8.75,-3.19,;-7.51,-2.28,;-7.98,-.82,;2.03,3.19,;3.57,3.19,)| Show InChI InChI=1S/C24H35N3O3/c28-23(16-19-4-5-19)25-20-8-6-18(7-9-20)10-11-26-12-14-27(15-13-26)21-2-1-3-22-24(21)30-17-29-22/h1-3,18-20H,4-17H2,(H,25,28)/t18-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121460
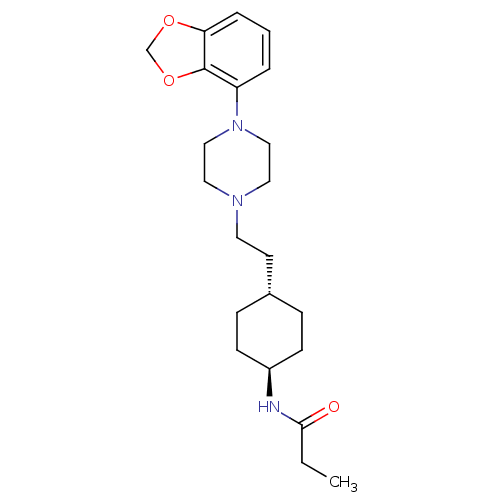
(US8722683, 7)Show SMILES CCC(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:5.4,wD:8.8,(9.63,1.85,;8.85,.52,;7.31,.52,;6.54,-.82,;6.54,1.85,;5,1.85,;4.23,.52,;2.69,.52,;1.93,1.85,;.38,1.85,;-.38,.52,;-1.93,.52,;-2.69,-.82,;-4.23,-.82,;-5,.52,;-4.23,1.85,;-2.69,1.85,;-6.54,.52,;-7.31,1.85,;-8.85,1.85,;-9.63,.52,;-8.85,-.82,;-9.33,-2.28,;-8.08,-3.19,;-6.84,-2.28,;-7.31,-.82,;2.69,3.19,;4.23,3.19,)| Show InChI InChI=1S/C22H33N3O3/c1-2-21(26)23-18-8-6-17(7-9-18)10-11-24-12-14-25(15-13-24)19-4-3-5-20-22(19)28-16-27-20/h3-5,17-18H,2,6-16H2,1H3,(H,23,26)/t17-,18- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121465
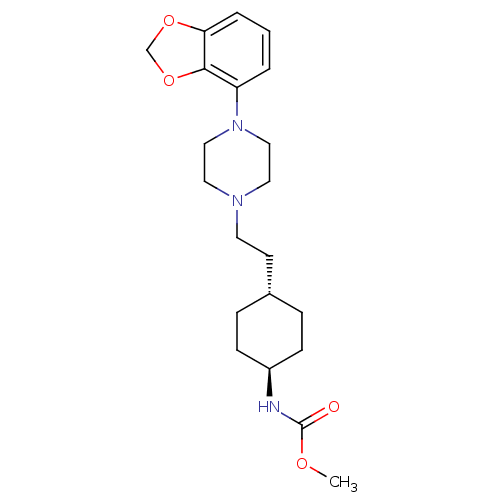
(US8722683, 12)Show SMILES COC(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:5.4,wD:8.8,(9.63,1.85,;8.86,.52,;7.32,.52,;6.55,-.82,;6.55,1.85,;5.01,1.85,;4.24,.52,;2.69,.52,;1.93,1.85,;.38,1.85,;-.39,.52,;-1.93,.52,;-2.69,-.82,;-4.24,-.82,;-5.01,.52,;-4.24,1.85,;-2.69,1.85,;-6.55,.52,;-7.32,1.85,;-8.86,1.85,;-9.63,.52,;-8.86,-.82,;-9.33,-2.28,;-8.09,-3.19,;-6.84,-2.28,;-7.32,-.82,;2.69,3.19,;4.24,3.19,)| Show InChI InChI=1S/C21H31N3O4/c1-26-21(25)22-17-7-5-16(6-8-17)9-10-23-11-13-24(14-12-23)18-3-2-4-19-20(18)28-15-27-19/h2-4,16-17H,5-15H2,1H3,(H,22,25)/t16-,17- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121530
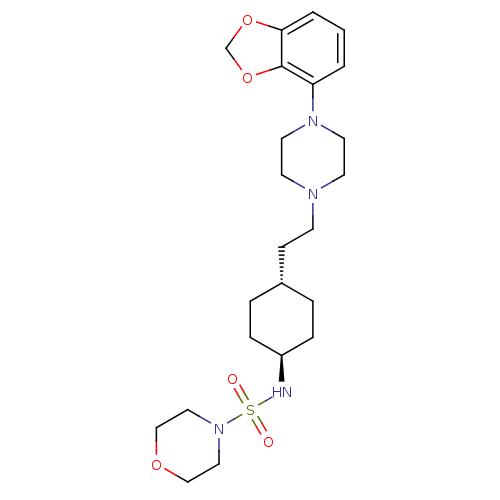
(US8722683, 77)Show SMILES O=S(=O)(N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1)N1CCOCC1 |r,wU:4.3,wD:7.7,(5.31,-.25,;6.64,.52,;7.98,1.29,;5.87,1.85,;4.33,1.85,;3.56,.52,;2.02,.52,;1.25,1.85,;-.29,1.85,;-1.06,.52,;-2.6,.52,;-3.37,-.82,;-4.91,-.82,;-5.68,.52,;-4.91,1.85,;-3.37,1.85,;-7.22,.52,;-7.99,1.85,;-9.53,1.85,;-10.3,.52,;-9.53,-.82,;-10,-2.28,;-8.76,-3.19,;-7.51,-2.28,;-7.99,-.82,;2.02,3.19,;3.56,3.19,;7.73,-.57,;7.33,-2.06,;8.44,-3.12,;9.91,-2.72,;10.3,-1.25,;9.22,-.17,)| Show InChI InChI=1S/C23H36N4O5S/c28-33(29,27-14-16-30-17-15-27)24-20-6-4-19(5-7-20)8-9-25-10-12-26(13-11-25)21-2-1-3-22-23(21)32-18-31-22/h1-3,19-20,24H,4-18H2/t19-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121466

(US8722683, 13)Show SMILES Cc1cc(CC(=O)N[C@H]2CC[C@H](CCN3CCN(CC3)c3cccc4OCOc34)CC2)on1 |r,wU:8.7,wD:11.11,(11.3,3.57,;9.97,2.8,;9.49,1.33,;7.95,1.33,;7.18,,;5.64,,;4.87,-1.33,;4.87,1.33,;3.33,1.33,;2.56,,;1.02,,;.25,1.33,;-1.29,1.33,;-2.06,,;-3.6,,;-4.37,-1.33,;-5.91,-1.33,;-6.68,,;-5.91,1.33,;-4.37,1.33,;-8.22,,;-8.99,1.33,;-10.53,1.33,;-11.3,,;-10.53,-1.33,;-11.01,-2.8,;-9.76,-3.7,;-8.51,-2.8,;-8.99,-1.33,;1.02,2.67,;2.56,2.67,;7.47,2.8,;8.72,3.7,)| Show InChI InChI=1S/C25H34N4O4/c1-18-15-21(33-27-18)16-24(30)26-20-7-5-19(6-8-20)9-10-28-11-13-29(14-12-28)22-3-2-4-23-25(22)32-17-31-23/h2-4,15,19-20H,5-14,16-17H2,1H3,(H,26,30)/t19-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121481
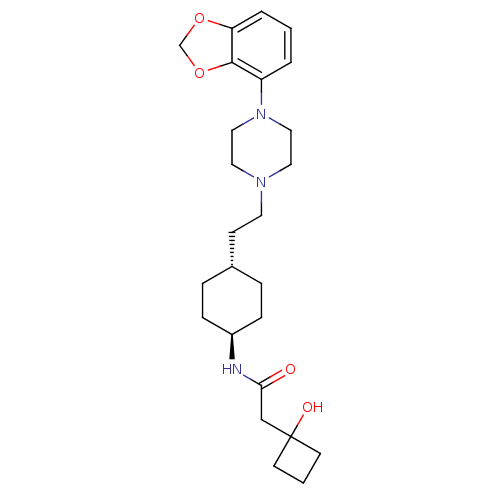
(US8722683, 28)Show SMILES OC1(CC(=O)N[C@H]2CC[C@H](CCN3CCN(CC3)c3cccc4OCOc34)CC2)CCC1 |r,wU:6.5,wD:9.9,(7.24,-1.59,;8.57,-.82,;7.8,.52,;6.26,.52,;5.49,-.82,;5.49,1.85,;3.95,1.85,;3.18,.52,;1.64,.52,;.87,1.85,;-.67,1.85,;-1.44,.52,;-2.98,.52,;-3.75,-.82,;-5.29,-.82,;-6.06,.52,;-5.29,1.85,;-3.75,1.85,;-7.6,.52,;-8.37,1.85,;-9.91,1.85,;-10.68,.52,;-9.91,-.82,;-10.38,-2.28,;-9.14,-3.19,;-7.89,-2.28,;-8.37,-.82,;1.64,3.19,;3.18,3.19,;9.34,-2.15,;10.68,-1.38,;9.91,-.05,)| Show InChI InChI=1S/C25H37N3O4/c29-23(17-25(30)10-2-11-25)26-20-7-5-19(6-8-20)9-12-27-13-15-28(16-14-27)21-3-1-4-22-24(21)32-18-31-22/h1,3-4,19-20,30H,2,5-18H2,(H,26,29)/t19-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121508
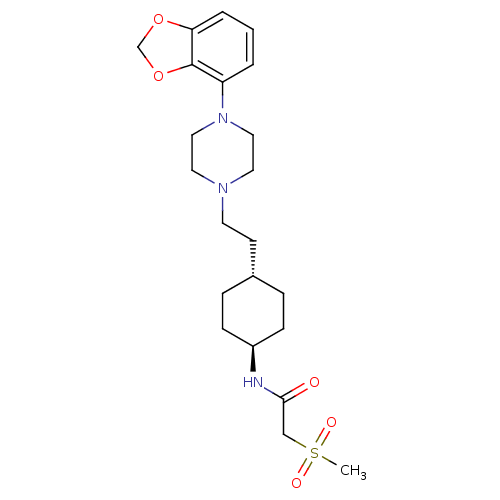
(US8722683, 55)Show SMILES CS(=O)(=O)CC(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:8.7,wD:11.11,(9.73,-2.15,;8.96,-.82,;7.62,-1.59,;10.29,-.05,;8.19,.52,;6.65,.52,;5.88,-.82,;5.88,1.85,;4.34,1.85,;3.57,.52,;2.03,.52,;1.26,1.85,;-.28,1.85,;-1.05,.52,;-2.59,.52,;-3.36,-.82,;-4.9,-.82,;-5.67,.52,;-4.9,1.85,;-3.36,1.85,;-7.21,.52,;-7.98,1.85,;-9.52,1.85,;-10.29,.52,;-9.52,-.82,;-10,-2.28,;-8.75,-3.19,;-7.51,-2.28,;-7.98,-.82,;2.03,3.19,;3.57,3.19,)| Show InChI InChI=1S/C22H33N3O5S/c1-31(27,28)15-21(26)23-18-7-5-17(6-8-18)9-10-24-11-13-25(14-12-24)19-3-2-4-20-22(19)30-16-29-20/h2-4,17-18H,5-16H2,1H3,(H,23,26)/t17-,18- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121480
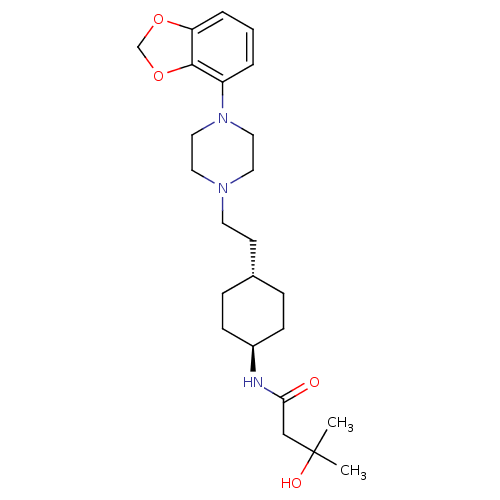
(US8722683, 27)Show SMILES CC(C)(O)CC(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:8.7,wD:11.11,(9.73,-2.15,;8.96,-.82,;7.62,-1.59,;10.29,-.05,;8.19,.52,;6.65,.52,;5.88,-.82,;5.88,1.85,;4.34,1.85,;3.57,.52,;2.03,.52,;1.26,1.85,;-.28,1.85,;-1.05,.52,;-2.59,.52,;-3.36,-.82,;-4.9,-.82,;-5.67,.52,;-4.9,1.85,;-3.36,1.85,;-7.21,.52,;-7.98,1.85,;-9.52,1.85,;-10.29,.52,;-9.52,-.82,;-10,-2.28,;-8.75,-3.19,;-7.51,-2.28,;-7.98,-.82,;2.03,3.19,;3.57,3.19,)| Show InChI InChI=1S/C24H37N3O4/c1-24(2,29)16-22(28)25-19-8-6-18(7-9-19)10-11-26-12-14-27(15-13-26)20-4-3-5-21-23(20)31-17-30-21/h3-5,18-19,29H,6-17H2,1-2H3,(H,25,28)/t18-,19- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121469

(US8722683, 16)Show SMILES FC(F)(F)CC(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:8.7,wD:11.11,(9.73,-2.15,;8.96,-.82,;10.29,-.05,;7.62,-1.59,;8.19,.52,;6.65,.52,;5.88,-.82,;5.88,1.85,;4.34,1.85,;3.57,.52,;2.03,.52,;1.26,1.85,;-.28,1.85,;-1.05,.52,;-2.59,.52,;-3.36,-.82,;-4.9,-.82,;-5.67,.52,;-4.9,1.85,;-3.36,1.85,;-7.21,.52,;-7.98,1.85,;-9.52,1.85,;-10.29,.52,;-9.52,-.82,;-10,-2.28,;-8.75,-3.19,;-7.51,-2.28,;-7.98,-.82,;2.03,3.19,;3.57,3.19,)| Show InChI InChI=1S/C22H30F3N3O3/c23-22(24,25)14-20(29)26-17-6-4-16(5-7-17)8-9-27-10-12-28(13-11-27)18-2-1-3-19-21(18)31-15-30-19/h1-3,16-17H,4-15H2,(H,26,29)/t16-,17- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121472
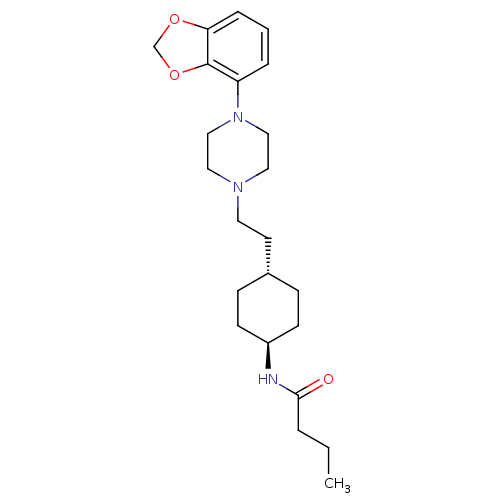
(US8722683, 19)Show SMILES CCCC(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:6.5,wD:9.9,(10.39,-.82,;8.85,-.82,;8.08,.52,;6.54,.52,;5.77,-.82,;5.77,1.85,;4.23,1.85,;3.46,.52,;1.92,.52,;1.15,1.85,;-.38,1.85,;-1.15,.52,;-2.69,.52,;-3.46,-.82,;-5,-.82,;-5.77,.52,;-5,1.85,;-3.46,1.85,;-7.31,.52,;-8.08,1.85,;-9.62,1.85,;-10.39,.52,;-9.62,-.82,;-10.1,-2.28,;-8.85,-3.19,;-7.61,-2.28,;-8.08,-.82,;1.92,3.19,;3.46,3.19,)| Show InChI InChI=1S/C23H35N3O3/c1-2-4-22(27)24-19-9-7-18(8-10-19)11-12-25-13-15-26(16-14-25)20-5-3-6-21-23(20)29-17-28-21/h3,5-6,18-19H,2,4,7-17H2,1H3,(H,24,27)/t18-,19- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121463

(US8722683, 10)Show SMILES OCC(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:5.4,wD:8.8,(9.63,1.85,;8.85,.52,;7.31,.52,;6.54,-.82,;6.54,1.85,;5,1.85,;4.23,.52,;2.69,.52,;1.93,1.85,;.38,1.85,;-.38,.52,;-1.93,.52,;-2.69,-.82,;-4.23,-.82,;-5,.52,;-4.23,1.85,;-2.69,1.85,;-6.54,.52,;-7.31,1.85,;-8.85,1.85,;-9.63,.52,;-8.85,-.82,;-9.33,-2.28,;-8.08,-3.19,;-6.84,-2.28,;-7.31,-.82,;2.69,3.19,;4.23,3.19,)| Show InChI InChI=1S/C21H31N3O4/c25-14-20(26)22-17-6-4-16(5-7-17)8-9-23-10-12-24(13-11-23)18-2-1-3-19-21(18)28-15-27-19/h1-3,16-17,25H,4-15H2,(H,22,26)/t16-,17- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121501

(US8722683, 48)Show SMILES O=C(N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1)c1cccnc1 |r,wU:3.2,wD:6.6,(5.39,-.82,;6.16,.52,;5.39,1.85,;3.85,1.85,;3.08,.52,;1.54,.52,;.77,1.85,;-.77,1.85,;-1.54,.52,;-3.08,.52,;-3.85,-.82,;-5.39,-.82,;-6.16,.52,;-5.39,1.85,;-3.85,1.85,;-7.7,.52,;-8.47,1.85,;-10.01,1.85,;-10.78,.52,;-10.01,-.82,;-10.49,-2.28,;-9.24,-3.19,;-7.99,-2.28,;-8.47,-.82,;1.54,3.19,;3.08,3.19,;7.7,.52,;8.47,-.82,;10.01,-.82,;10.78,.52,;10.01,1.85,;8.47,1.85,)| Show InChI InChI=1S/C25H32N4O3/c30-25(20-3-2-11-26-17-20)27-21-8-6-19(7-9-21)10-12-28-13-15-29(16-14-28)22-4-1-5-23-24(22)32-18-31-23/h1-5,11,17,19,21H,6-10,12-16,18H2,(H,27,30)/t19-,21- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121470
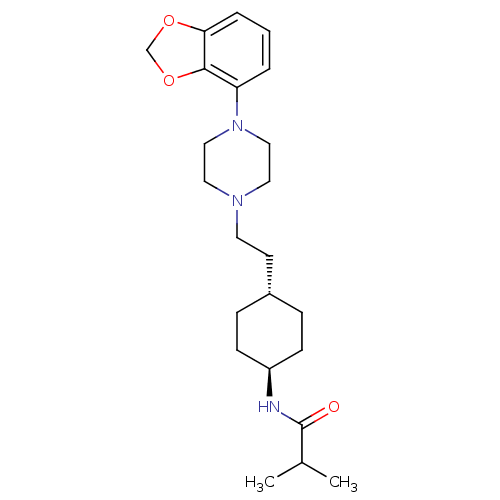
(US8722683, 17)Show SMILES CC(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:6.5,wD:9.9,(7.7,-2.15,;6.93,-.82,;5.39,-.82,;7.7,.52,;9.24,.52,;6.93,1.85,;5.39,1.85,;4.62,.52,;3.08,.52,;2.31,1.85,;.77,1.85,;,.52,;-1.54,.52,;-2.31,-.82,;-3.85,-.82,;-4.62,.52,;-3.85,1.85,;-2.31,1.85,;-6.16,.52,;-6.93,1.85,;-8.47,1.85,;-9.24,.52,;-8.47,-.82,;-8.95,-2.28,;-7.7,-3.19,;-6.45,-2.28,;-6.93,-.82,;3.08,3.19,;4.62,3.19,)| Show InChI InChI=1S/C23H35N3O3/c1-17(2)23(27)24-19-8-6-18(7-9-19)10-11-25-12-14-26(15-13-25)20-4-3-5-21-22(20)29-16-28-21/h3-5,17-19H,6-16H2,1-2H3,(H,24,27)/t18-,19- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121526

(US8722683, 73)Show SMILES FCC(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:5.4,wD:8.8,(9.63,1.85,;8.85,.52,;7.31,.52,;6.54,-.82,;6.54,1.85,;5,1.85,;4.23,.52,;2.69,.52,;1.93,1.85,;.38,1.85,;-.38,.52,;-1.93,.52,;-2.69,-.82,;-4.23,-.82,;-5,.52,;-4.23,1.85,;-2.69,1.85,;-6.54,.52,;-7.31,1.85,;-8.85,1.85,;-9.63,.52,;-8.85,-.82,;-9.33,-2.28,;-8.08,-3.19,;-6.84,-2.28,;-7.31,-.82,;2.69,3.19,;4.23,3.19,)| Show InChI InChI=1S/C21H30FN3O3/c22-14-20(26)23-17-6-4-16(5-7-17)8-9-24-10-12-25(13-11-24)18-2-1-3-19-21(18)28-15-27-19/h1-3,16-17H,4-15H2,(H,23,26)/t16-,17- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121482

(US8722683, 29)Show SMILES OC1(CC1)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:7.7,wD:10.11,(9.27,-.82,;8.5,.52,;9.98,.92,;8.89,2.01,;6.96,.52,;6.19,-.82,;6.19,1.85,;4.65,1.85,;3.88,.52,;2.34,.52,;1.57,1.85,;.03,1.85,;-.74,.52,;-2.28,.52,;-3.05,-.82,;-4.59,-.82,;-5.36,.52,;-4.59,1.85,;-3.05,1.85,;-6.9,.52,;-7.67,1.85,;-9.21,1.85,;-9.98,.52,;-9.21,-.82,;-9.69,-2.28,;-8.44,-3.19,;-7.2,-2.28,;-7.67,-.82,;2.34,3.19,;3.88,3.19,)| Show InChI InChI=1S/C23H33N3O4/c27-22(23(28)9-10-23)24-18-6-4-17(5-7-18)8-11-25-12-14-26(15-13-25)19-2-1-3-20-21(19)30-16-29-20/h1-3,17-18,28H,4-16H2,(H,24,27)/t17-,18- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121519

(US8722683, 66)Show SMILES FC(F)(F)CS(=O)(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:9.8,wD:12.12,(8.08,3.19,;8.85,1.85,;10.39,1.85,;7.52,1.08,;8.08,.52,;6.54,.52,;5.77,-.82,;6.54,-1.02,;5.77,1.85,;4.23,1.85,;3.46,.52,;1.92,.52,;1.15,1.85,;-.38,1.85,;-1.15,.52,;-2.7,.52,;-3.46,-.82,;-5,-.82,;-5.77,.52,;-5,1.85,;-3.46,1.85,;-7.31,.52,;-8.08,1.85,;-9.62,1.85,;-10.39,.52,;-9.62,-.82,;-10.1,-2.28,;-8.85,-3.19,;-7.61,-2.28,;-8.08,-.82,;1.92,3.19,;3.46,3.19,)| Show InChI InChI=1S/C21H30F3N3O4S/c22-21(23,24)14-32(28,29)25-17-6-4-16(5-7-17)8-9-26-10-12-27(13-11-26)18-2-1-3-19-20(18)31-15-30-19/h1-3,16-17,25H,4-15H2/t16-,17- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121455

(US8722683, 2)Show SMILES COCCC(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:7.6,wD:10.10,(10.78,-2.15,;10.01,-.82,;8.47,-.82,;7.7,.52,;6.16,.52,;5.39,-.82,;5.39,1.85,;3.85,1.85,;3.08,.52,;1.54,.52,;.77,1.85,;-.77,1.85,;-1.54,.52,;-3.08,.52,;-3.85,-.82,;-5.39,-.82,;-6.16,.52,;-5.39,1.85,;-3.85,1.85,;-7.7,.52,;-8.47,1.85,;-10.01,1.85,;-10.78,.52,;-10.01,-.82,;-10.49,-2.28,;-9.24,-3.19,;-7.99,-2.28,;-8.47,-.82,;1.54,3.19,;3.08,3.19,)| Show InChI InChI=1S/C23H35N3O4/c1-28-16-10-22(27)24-19-7-5-18(6-8-19)9-11-25-12-14-26(15-13-25)20-3-2-4-21-23(20)30-17-29-21/h2-4,18-19H,5-17H2,1H3,(H,24,27)/t18-,19- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121507

(US8722683, 54)Show SMILES O=C(N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1)C1CCC1 |r,wU:3.2,wD:6.6,(5.84,-.82,;6.61,.52,;5.84,1.85,;4.3,1.85,;3.53,.52,;1.99,.52,;1.22,1.85,;-.32,1.85,;-1.09,.52,;-2.63,.52,;-3.4,-.82,;-4.94,-.82,;-5.71,.52,;-4.94,1.85,;-3.4,1.85,;-7.25,.52,;-8.02,1.85,;-9.56,1.85,;-10.33,.52,;-9.56,-.82,;-10.03,-2.28,;-8.79,-3.19,;-7.54,-2.28,;-8.02,-.82,;1.99,3.19,;3.53,3.19,;8.15,.52,;9.24,-.57,;10.33,.52,;9.24,1.61,)| Show InChI InChI=1S/C24H35N3O3/c28-24(19-3-1-4-19)25-20-9-7-18(8-10-20)11-12-26-13-15-27(16-14-26)21-5-2-6-22-23(21)30-17-29-22/h2,5-6,18-20H,1,3-4,7-17H2,(H,25,28)/t18-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121524

(US8722683, 71)Show SMILES O=C(N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1)N1CCc2ccccc12 |r,wU:3.2,wD:6.6,(5.17,-.78,;5.94,.56,;5.17,1.89,;3.63,1.89,;2.86,.56,;1.32,.56,;.55,1.89,;-.99,1.89,;-1.76,.56,;-3.3,.56,;-4.07,-.78,;-5.61,-.78,;-6.38,.56,;-5.61,1.89,;-4.07,1.89,;-7.92,.56,;-8.69,1.89,;-10.23,1.89,;-11,.56,;-10.23,-.78,;-10.7,-2.24,;-9.46,-3.15,;-8.21,-2.24,;-8.69,-.78,;1.32,3.23,;2.86,3.23,;7.48,.56,;8.39,1.8,;9.85,1.33,;9.85,-.21,;11,-1.24,;10.68,-2.75,;9.21,-3.23,;8.07,-2.19,;8.39,-.69,)| Show InChI InChI=1S/C28H36N4O3/c33-28(32-15-13-22-4-1-2-5-24(22)32)29-23-10-8-21(9-11-23)12-14-30-16-18-31(19-17-30)25-6-3-7-26-27(25)35-20-34-26/h1-7,21,23H,8-20H2,(H,29,33)/t21-,23- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 2.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121475

(US8722683, 22)Show SMILES O=C(CC1COC1)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:8.8,wD:11.12,(5.8,-.82,;6.57,.52,;8.11,.52,;8.88,-.82,;8.48,-2.3,;9.97,-2.7,;10.37,-1.21,;5.8,1.85,;4.26,1.85,;3.49,.52,;1.95,.52,;1.18,1.85,;-.36,1.85,;-1.13,.52,;-2.67,.52,;-3.44,-.82,;-4.98,-.82,;-5.75,.52,;-4.98,1.85,;-3.44,1.85,;-7.29,.52,;-8.06,1.85,;-9.6,1.85,;-10.37,.52,;-9.6,-.82,;-10.07,-2.28,;-8.83,-3.19,;-7.58,-2.28,;-8.06,-.82,;1.95,3.19,;3.49,3.19,)| Show InChI InChI=1S/C24H35N3O4/c28-23(14-19-15-29-16-19)25-20-6-4-18(5-7-20)8-9-26-10-12-27(13-11-26)21-2-1-3-22-24(21)31-17-30-22/h1-3,18-20H,4-17H2,(H,25,28)/t18-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2.06 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121506
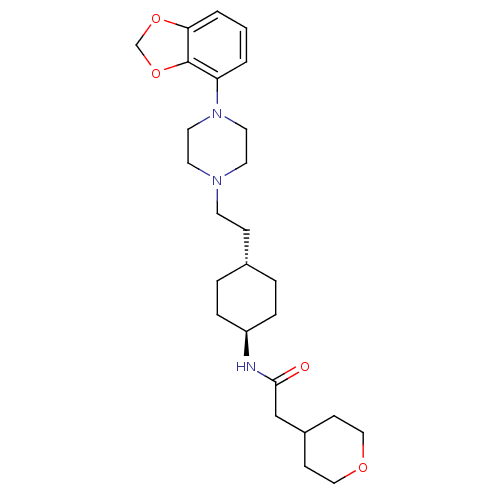
(US8722683, 53)Show SMILES O=C(CC1CCOCC1)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:10.10,wD:13.14,(5.39,-1.48,;6.16,-.15,;7.7,-.15,;8.47,1.18,;10.01,1.18,;10.78,2.52,;10.01,3.85,;8.47,3.85,;7.7,2.52,;5.39,1.18,;3.85,1.18,;3.08,-.15,;1.54,-.15,;.77,1.18,;-.77,1.18,;-1.54,-.15,;-3.08,-.15,;-3.85,-1.48,;-5.39,-1.48,;-6.16,-.15,;-5.39,1.18,;-3.85,1.18,;-7.7,-.15,;-8.47,1.18,;-10.01,1.18,;-10.78,-.15,;-10.01,-1.48,;-10.49,-2.95,;-9.24,-3.85,;-7.99,-2.95,;-8.47,-1.48,;1.54,2.52,;3.08,2.52,)| Show InChI InChI=1S/C26H39N3O4/c30-25(18-21-9-16-31-17-10-21)27-22-6-4-20(5-7-22)8-11-28-12-14-29(15-13-28)23-2-1-3-24-26(23)33-19-32-24/h1-3,20-22H,4-19H2,(H,27,30)/t20-,22- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2.09 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121504
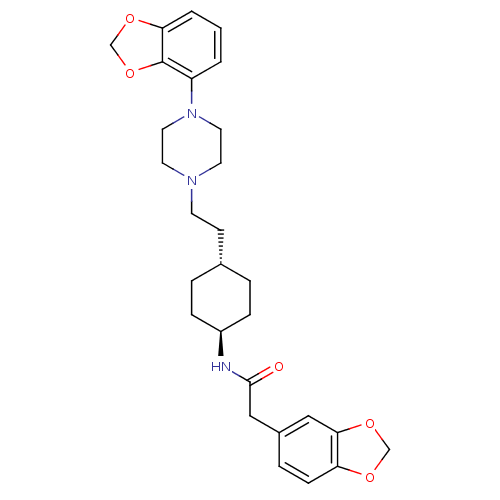
(US8722683, 51)Show SMILES O=C(Cc1ccc2OCOc2c1)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:13.14,wD:16.18,(4.56,-2.05,;5.33,-.72,;6.87,-.72,;7.64,.61,;6.87,1.95,;7.64,3.28,;9.18,3.28,;10.21,4.42,;11.61,3.8,;11.45,2.27,;9.95,1.95,;9.18,.61,;4.56,.61,;3.02,.61,;2.25,-.72,;.71,-.72,;-.06,.61,;-1.6,.61,;-2.37,-.72,;-3.91,-.72,;-4.68,-2.05,;-6.22,-2.05,;-6.99,-.72,;-6.22,.61,;-4.68,.61,;-8.53,-.72,;-9.3,.61,;-10.84,.61,;-11.61,-.72,;-10.84,-2.05,;-11.32,-3.52,;-10.07,-4.42,;-8.83,-3.52,;-9.3,-2.05,;.71,1.95,;2.25,1.95,)| Show InChI InChI=1S/C28H35N3O5/c32-27(17-21-6-9-24-26(16-21)35-18-33-24)29-22-7-4-20(5-8-22)10-11-30-12-14-31(15-13-30)23-2-1-3-25-28(23)36-19-34-25/h1-3,6,9,16,20,22H,4-5,7-8,10-15,17-19H2,(H,29,32)/t20-,22- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 2.11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121495

(US8722683, 42)Show SMILES C[C@@H](O)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:6.5,1.1,wD:9.9,(9.63,1.85,;8.85,.52,;9.63,-.82,;7.32,.52,;6.55,-.82,;6.55,1.85,;5,1.85,;4.23,.52,;2.7,.52,;1.93,1.85,;.38,1.85,;-.38,.52,;-1.92,.52,;-2.69,-.82,;-4.23,-.82,;-5,.52,;-4.23,1.85,;-2.69,1.85,;-6.55,.52,;-7.31,1.85,;-8.85,1.85,;-9.63,.52,;-8.85,-.82,;-9.33,-2.28,;-8.08,-3.19,;-6.84,-2.28,;-7.31,-.82,;2.7,3.19,;4.23,3.19,)| Show InChI InChI=1S/C22H33N3O4/c1-16(26)22(27)23-18-7-5-17(6-8-18)9-10-24-11-13-25(14-12-24)19-3-2-4-20-21(19)29-15-28-20/h2-4,16-18,26H,5-15H2,1H3,(H,23,27)/t16-,17-,18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121521

(US8722683, 68)Show SMILES Fc1ccc(cc1)S(=O)(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:11.11,wD:14.15,(10.65,-3.51,;9.56,-2.42,;8.07,-2.82,;6.98,-1.73,;7.38,-.24,;8.87,.15,;9.96,-.94,;6.29,.84,;4.96,.07,;7.63,1.61,;5.52,2.18,;3.98,2.18,;3.21,.84,;1.67,.84,;.9,2.18,;-.64,2.18,;-1.41,.84,;-2.95,.84,;-3.72,-.49,;-5.26,-.49,;-6.03,.84,;-5.26,2.18,;-3.72,2.18,;-7.57,.84,;-8.34,2.18,;-9.88,2.18,;-10.65,.84,;-9.88,-.49,;-10.35,-1.95,;-9.11,-2.86,;-7.86,-1.95,;-8.34,-.49,;1.67,3.51,;3.21,3.51,)| Show InChI InChI=1S/C25H32FN3O4S/c26-20-6-10-22(11-7-20)34(30,31)27-21-8-4-19(5-9-21)12-13-28-14-16-29(17-15-28)23-2-1-3-24-25(23)33-18-32-24/h1-3,6-7,10-11,19,21,27H,4-5,8-9,12-18H2/t19-,21- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121522

(US8722683, 69)Show SMILES Cn1cnc(c1)S(=O)(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:10.10,wD:13.14,(10.87,-2.11,;9.53,-1.34,;8.63,-2.59,;7.16,-2.11,;7.16,-.57,;8.63,-.1,;6.07,.52,;4.74,-.25,;7.41,1.29,;5.3,1.85,;3.76,1.85,;2.99,.52,;1.45,.52,;.68,1.85,;-.86,1.85,;-1.63,.52,;-3.17,.52,;-3.94,-.82,;-5.48,-.82,;-6.25,.52,;-5.48,1.85,;-3.94,1.85,;-7.79,.52,;-8.56,1.85,;-10.1,1.85,;-10.87,.52,;-10.1,-.82,;-10.57,-2.28,;-9.33,-3.19,;-8.08,-2.28,;-8.56,-.82,;1.45,3.19,;2.99,3.19,)| Show InChI InChI=1S/C23H33N5O4S/c1-26-15-22(24-16-26)33(29,30)25-19-7-5-18(6-8-19)9-10-27-11-13-28(14-12-27)20-3-2-4-21-23(20)32-17-31-21/h2-4,15-16,18-19,25H,5-14,17H2,1H3/t18-,19- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 2.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121471

(US8722683, 18)Show SMILES CC(C)CC(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:7.6,wD:10.10,(8.08,-2.15,;8.85,-.82,;10.39,-.82,;8.08,.52,;6.54,.52,;5.77,-.82,;5.77,1.85,;4.23,1.85,;3.46,.52,;1.92,.52,;1.15,1.85,;-.38,1.85,;-1.15,.52,;-2.69,.52,;-3.46,-.82,;-5,-.82,;-5.77,.52,;-5,1.85,;-3.46,1.85,;-7.31,.52,;-8.08,1.85,;-9.62,1.85,;-10.39,.52,;-9.62,-.82,;-10.1,-2.28,;-8.85,-3.19,;-7.61,-2.28,;-8.08,-.82,;1.92,3.19,;3.46,3.19,)| Show InChI InChI=1S/C24H37N3O3/c1-18(2)16-23(28)25-20-8-6-19(7-9-20)10-11-26-12-14-27(15-13-26)21-4-3-5-22-24(21)30-17-29-22/h3-5,18-20H,6-17H2,1-2H3,(H,25,28)/t19-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2.28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121500

(US8722683, 47)Show SMILES O=C(N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1)c1ccnc2ccccc12 |r,wU:3.2,wD:6.6,(5.39,-.67,;6.16,.67,;5.39,2,;3.85,2,;3.08,.67,;1.54,.67,;.77,2,;-.77,2,;-1.54,.67,;-3.08,.67,;-3.85,-.67,;-5.39,-.67,;-6.16,.67,;-5.39,2,;-3.85,2,;-7.7,.67,;-8.47,2,;-10.01,2,;-10.78,.67,;-10.01,-.67,;-10.49,-2.13,;-9.24,-3.04,;-7.99,-2.13,;-8.47,-.67,;1.54,3.33,;3.08,3.33,;7.7,.67,;8.47,2,;10.01,2,;10.78,.67,;10.01,-.67,;10.78,-2,;10.01,-3.33,;8.47,-3.33,;7.7,-2,;8.47,-.67,)| Show InChI InChI=1S/C29H34N4O3/c34-29(24-12-14-30-25-5-2-1-4-23(24)25)31-22-10-8-21(9-11-22)13-15-32-16-18-33(19-17-32)26-6-3-7-27-28(26)36-20-35-27/h1-7,12,14,21-22H,8-11,13,15-20H2,(H,31,34)/t21-,22- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121527
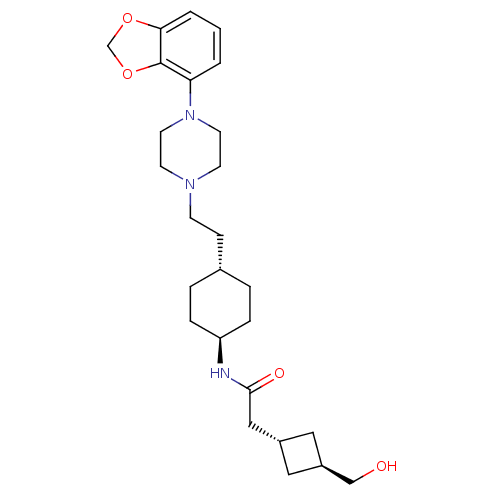
(US8722683, 74)Show SMILES OC[C@H]1C[C@H](CC(=O)N[C@H]2CC[C@H](CCN3CCN(CC3)c3cccc4OCOc34)CC2)C1 |r,wU:9.8,2.1,wD:12.12,4.4,(11.28,4.13,;9.83,4.13,;9.06,2.79,;9.46,1.31,;7.97,.91,;7.2,-.43,;5.66,-.43,;4.89,-1.76,;4.89,.91,;3.35,.91,;2.58,-.43,;1.04,-.43,;.27,.91,;-1.27,.91,;-2.04,-.43,;-3.58,-.43,;-4.35,-1.76,;-5.89,-1.76,;-6.66,-.43,;-5.89,.91,;-4.35,.91,;-8.2,-.43,;-8.97,.91,;-10.51,.91,;-11.28,-.43,;-10.51,-1.76,;-10.99,-3.22,;-9.74,-4.13,;-8.49,-3.22,;-8.97,-1.76,;1.04,2.24,;2.58,2.24,;7.57,2.4,)| Show InChI InChI=1S/C26H39N3O4/c30-17-21-14-20(15-21)16-25(31)27-22-6-4-19(5-7-22)8-9-28-10-12-29(13-11-28)23-2-1-3-24-26(23)33-18-32-24/h1-3,19-22,30H,4-18H2,(H,27,31)/t19-,20-,21-,22- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2.44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121476

(US8722683, 23)Show SMILES CO[C@@H]1CC[C@@H](CC(=O)N[C@H]2CC[C@H](CCN3CCN(CC3)c3cccc4OCOc34)CC2)C1 |r,wU:10.9,5.5,wD:13.13,2.1,(11.3,-3.89,;11.3,-2.35,;9.97,-1.58,;8.72,-2.48,;7.47,-1.58,;7.95,-.11,;7.18,1.22,;5.64,1.22,;4.87,-.11,;4.87,2.55,;3.33,2.55,;2.56,1.22,;1.02,1.22,;.25,2.55,;-1.29,2.55,;-2.06,1.22,;-3.6,1.22,;-4.37,-.11,;-5.91,-.11,;-6.68,1.22,;-5.91,2.55,;-4.37,2.55,;-8.22,1.22,;-8.99,2.55,;-10.53,2.55,;-11.3,1.22,;-10.53,-.11,;-11.01,-1.58,;-9.76,-2.48,;-8.51,-1.58,;-8.99,-.11,;1.02,3.89,;2.56,3.89,;9.49,-.11,)| Show InChI InChI=1S/C27H41N3O4/c1-32-23-10-7-21(17-23)18-26(31)28-22-8-5-20(6-9-22)11-12-29-13-15-30(16-14-29)24-3-2-4-25-27(24)34-19-33-25/h2-4,20-23H,5-19H2,1H3,(H,28,31)/t20-,21-,22-,23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121458

(US8722683, 5)Show SMILES O=C(C[C@@H]1COCCO1)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:10.10,wD:13.14,3.2,(5.39,-.67,;6.16,.67,;7.7,.67,;8.47,-.67,;10.01,-.67,;10.78,-2,;10.01,-3.33,;8.47,-3.33,;7.7,-2,;5.39,2,;3.85,2,;3.08,.67,;1.54,.67,;.77,2,;-.77,2,;-1.54,.67,;-3.08,.67,;-3.85,-.67,;-5.39,-.67,;-6.16,.67,;-5.39,2,;-3.85,2,;-7.7,.67,;-8.47,2,;-10.01,2,;-10.78,.67,;-10.01,-.67,;-10.49,-2.13,;-9.24,-3.04,;-7.99,-2.13,;-8.47,-.67,;1.54,3.33,;3.08,3.33,)| Show InChI InChI=1S/C25H37N3O5/c29-24(16-21-17-30-14-15-31-21)26-20-6-4-19(5-7-20)8-9-27-10-12-28(13-11-27)22-2-1-3-23-25(22)33-18-32-23/h1-3,19-21H,4-18H2,(H,26,29)/t19-,20-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121473

(US8722683, 20)Show SMILES FC1(F)CC1C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:8.8,wD:11.12,(10.29,-1.59,;9.52,-.25,;8.75,-1.59,;9.52,1.29,;8.19,.52,;6.65,.52,;5.88,-.82,;5.88,1.85,;4.34,1.85,;3.57,.52,;2.03,.52,;1.26,1.85,;-.28,1.85,;-1.05,.52,;-2.59,.52,;-3.36,-.82,;-4.9,-.82,;-5.67,.52,;-4.9,1.85,;-3.36,1.85,;-7.21,.52,;-7.98,1.85,;-9.52,1.85,;-10.29,.52,;-9.52,-.82,;-10,-2.28,;-8.75,-3.19,;-7.51,-2.28,;-7.98,-.82,;2.03,3.19,;3.57,3.19,)| Show InChI InChI=1S/C23H31F2N3O3/c24-23(25)14-18(23)22(29)26-17-6-4-16(5-7-17)8-9-27-10-12-28(13-11-27)19-2-1-3-20-21(19)31-15-30-20/h1-3,16-18H,4-15H2,(H,26,29)/t16-,17-,18? | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121456
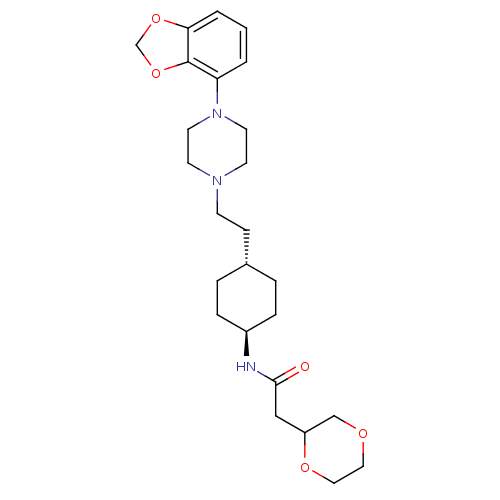
(US8722683, 3)Show SMILES O=C(CC1COCCO1)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:10.10,wD:13.14,(5.39,-.67,;6.16,.67,;7.7,.67,;8.47,-.67,;10.01,-.67,;10.78,-2,;10.01,-3.33,;8.47,-3.33,;7.7,-2,;5.39,2,;3.85,2,;3.08,.67,;1.54,.67,;.77,2,;-.77,2,;-1.54,.67,;-3.08,.67,;-3.85,-.67,;-5.39,-.67,;-6.16,.67,;-5.39,2,;-3.85,2,;-7.7,.67,;-8.47,2,;-10.01,2,;-10.78,.67,;-10.01,-.67,;-10.49,-2.13,;-9.24,-3.04,;-7.99,-2.13,;-8.47,-.67,;1.54,3.33,;3.08,3.33,)| Show InChI InChI=1S/C25H37N3O5/c29-24(16-21-17-30-14-15-31-21)26-20-6-4-19(5-7-20)8-9-27-10-12-28(13-11-27)22-2-1-3-23-25(22)33-18-32-23/h1-3,19-21H,4-18H2,(H,26,29)/t19-,20-,21? | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2.56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121474

(US8722683, 21)Show SMILES COC(C)CC(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:8.7,wD:11.11,(10.78,.52,;10.01,-.82,;8.47,-.82,;7.7,-2.15,;7.7,.52,;6.16,.52,;5.39,-.82,;5.39,1.85,;3.85,1.85,;3.08,.52,;1.54,.52,;.77,1.85,;-.77,1.85,;-1.54,.52,;-3.08,.52,;-3.85,-.82,;-5.39,-.82,;-6.16,.52,;-5.39,1.85,;-3.85,1.85,;-7.7,.52,;-8.47,1.85,;-10.01,1.85,;-10.78,.52,;-10.01,-.82,;-10.49,-2.28,;-9.24,-3.19,;-7.99,-2.28,;-8.47,-.82,;1.54,3.19,;3.08,3.19,)| Show InChI InChI=1S/C24H37N3O4/c1-18(29-2)16-23(28)25-20-8-6-19(7-9-20)10-11-26-12-14-27(15-13-26)21-4-3-5-22-24(21)31-17-30-22/h3-5,18-20H,6-17H2,1-2H3,(H,25,28)/t18?,19-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2.76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121457

(US8722683, 4)Show SMILES O=C(C[C@H]1COCCO1)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:10.10,3.2,wD:13.14,(5.39,-.67,;6.16,.67,;7.7,.67,;8.47,-.67,;10.01,-.67,;10.78,-2,;10.01,-3.33,;8.47,-3.33,;7.7,-2,;5.39,2,;3.85,2,;3.08,.67,;1.54,.67,;.77,2,;-.77,2,;-1.54,.67,;-3.08,.67,;-3.85,-.67,;-5.39,-.67,;-6.16,.67,;-5.39,2,;-3.85,2,;-7.7,.67,;-8.47,2,;-10.01,2,;-10.78,.67,;-10.01,-.67,;-10.49,-2.13,;-9.24,-3.04,;-7.99,-2.13,;-8.47,-.67,;1.54,3.33,;3.08,3.33,)| Show InChI InChI=1S/C25H37N3O5/c29-24(16-21-17-30-14-15-31-21)26-20-6-4-19(5-7-20)8-9-27-10-12-28(13-11-27)22-2-1-3-23-25(22)33-18-32-23/h1-3,19-21H,4-18H2,(H,26,29)/t19-,20-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2.85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121520

(US8722683, 67)Show SMILES O=S(=O)(N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1)c1ccccc1 |r,wU:4.3,wD:7.7,(5.3,-.25,;6.64,.52,;7.97,1.29,;5.87,1.85,;4.33,1.85,;3.56,.52,;2.02,.52,;1.25,1.85,;-.29,1.85,;-1.06,.52,;-2.6,.52,;-3.37,-.82,;-4.91,-.82,;-5.68,.52,;-4.91,1.85,;-3.37,1.85,;-7.22,.52,;-7.99,1.85,;-9.53,1.85,;-10.3,.52,;-9.53,-.82,;-10.01,-2.28,;-8.76,-3.19,;-7.52,-2.28,;-7.99,-.82,;2.02,3.19,;3.56,3.19,;7.73,-.57,;7.33,-2.06,;8.42,-3.15,;9.9,-2.75,;10.3,-1.26,;9.21,-.17,)| Show InChI InChI=1S/C25H33N3O4S/c29-33(30,22-5-2-1-3-6-22)26-21-11-9-20(10-12-21)13-14-27-15-17-28(18-16-27)23-7-4-8-24-25(23)32-19-31-24/h1-8,20-21,26H,9-19H2/t20-,21- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2.88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121513

(US8722683, 60)Show SMILES ClC1CC(C1)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:8.8,wD:11.12,(11.1,.52,;9.56,.52,;8.47,1.61,;7.38,.52,;8.47,-.57,;5.84,.52,;5.07,-.82,;5.07,1.85,;3.53,1.85,;2.76,.52,;1.22,.52,;.45,1.85,;-1.09,1.85,;-1.86,.52,;-3.4,.52,;-4.17,-.82,;-5.71,-.82,;-6.48,.52,;-5.71,1.85,;-4.17,1.85,;-8.02,.52,;-8.79,1.85,;-10.33,1.85,;-11.1,.52,;-10.33,-.82,;-10.81,-2.28,;-9.56,-3.19,;-8.31,-2.28,;-8.79,-.82,;1.22,3.19,;2.76,3.19,)| Show InChI InChI=1S/C24H34ClN3O3/c25-19-14-18(15-19)24(29)26-20-6-4-17(5-7-20)8-9-27-10-12-28(13-11-27)21-2-1-3-22-23(21)31-16-30-22/h1-3,17-20H,4-16H2,(H,26,29)/t17-,18?,19?,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121485
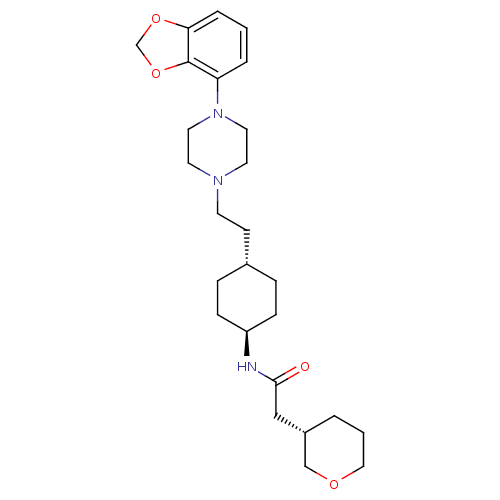
(US8722683, 32)Show SMILES O=C(C[C@@H]1CCCOC1)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:10.10,wD:13.14,3.2,(5.39,-.67,;6.16,.67,;7.7,.67,;8.47,-.67,;10.01,-.67,;10.78,-2,;10.01,-3.33,;8.47,-3.33,;7.7,-2,;5.39,2,;3.85,2,;3.08,.67,;1.54,.67,;.77,2,;-.77,2,;-1.54,.67,;-3.08,.67,;-3.85,-.67,;-5.39,-.67,;-6.16,.67,;-5.39,2,;-3.85,2,;-7.7,.67,;-8.47,2,;-10.01,2,;-10.78,.67,;-10.01,-.67,;-10.49,-2.13,;-9.24,-3.04,;-7.99,-2.13,;-8.47,-.67,;1.54,3.33,;3.08,3.33,)| Show InChI InChI=1S/C26H39N3O4/c30-25(17-21-3-2-16-31-18-21)27-22-8-6-20(7-9-22)10-11-28-12-14-29(15-13-28)23-4-1-5-24-26(23)33-19-32-24/h1,4-5,20-22H,2-3,6-19H2,(H,27,30)/t20-,21-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2.99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121459

(US8722683, 6)Show SMILES O=C(CC1CCCO1)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:9.9,wD:12.13,(5.54,-.82,;6.31,.52,;7.85,.52,;8.62,-.82,;10.16,-.82,;10.63,-2.28,;9.39,-3.19,;8.14,-2.28,;5.54,1.85,;4,1.85,;3.23,.52,;1.69,.52,;.92,1.85,;-.62,1.85,;-1.39,.52,;-2.93,.52,;-3.7,-.82,;-5.24,-.82,;-6.01,.52,;-5.24,1.85,;-3.7,1.85,;-7.55,.52,;-8.32,1.85,;-9.86,1.85,;-10.63,.52,;-9.86,-.82,;-10.34,-2.28,;-9.09,-3.19,;-7.85,-2.28,;-8.32,-.82,;1.69,3.19,;3.23,3.19,)| Show InChI InChI=1S/C25H37N3O4/c29-24(17-21-3-2-16-30-21)26-20-8-6-19(7-9-20)10-11-27-12-14-28(15-13-27)22-4-1-5-23-25(22)32-18-31-23/h1,4-5,19-21H,2-3,6-18H2,(H,26,29)/t19-,20-,21? | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121487

(US8722683, 34)Show SMILES CS(=O)(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:5.4,wD:8.8,(8.57,-.82,;7.8,.52,;6.47,-.25,;9.14,1.29,;7.03,1.85,;5.49,1.85,;4.72,.52,;3.18,.52,;2.41,1.85,;.87,1.85,;.1,.52,;-1.44,.52,;-2.21,-.82,;-3.75,-.82,;-4.52,.52,;-3.75,1.85,;-2.21,1.85,;-6.06,.52,;-6.83,1.85,;-8.37,1.85,;-9.14,.52,;-8.37,-.82,;-8.84,-2.28,;-7.6,-3.19,;-6.35,-2.28,;-6.83,-.82,;3.18,3.19,;4.72,3.19,)| Show InChI InChI=1S/C20H31N3O4S/c1-28(24,25)21-17-7-5-16(6-8-17)9-10-22-11-13-23(14-12-22)18-3-2-4-19-20(18)27-15-26-19/h2-4,16-17,21H,5-15H2,1H3/t16-,17- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121529
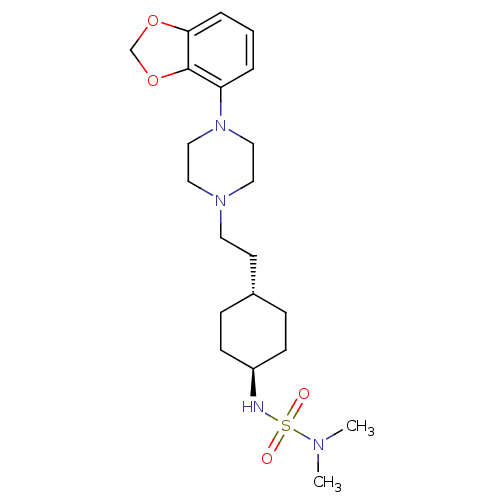
(US8722683, 76)Show SMILES CN(C)S(=O)(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:7.6,wD:10.10,(9.76,-.17,;8.27,-.57,;7.87,-2.06,;7.18,.52,;5.85,-.25,;8.52,1.29,;6.41,1.85,;4.87,1.85,;4.1,.52,;2.56,.52,;1.79,1.85,;.25,1.85,;-.52,.52,;-2.06,.52,;-2.83,-.82,;-4.37,-.82,;-5.14,.52,;-4.37,1.85,;-2.83,1.85,;-6.68,.52,;-7.45,1.85,;-8.99,1.85,;-9.76,.52,;-8.99,-.82,;-9.46,-2.28,;-8.22,-3.19,;-6.97,-2.28,;-7.45,-.82,;2.56,3.19,;4.1,3.19,)| Show InChI InChI=1S/C21H34N4O4S/c1-23(2)30(26,27)22-18-8-6-17(7-9-18)10-11-24-12-14-25(15-13-24)19-4-3-5-20-21(19)29-16-28-20/h3-5,17-18,22H,6-16H2,1-2H3/t17-,18- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 3.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121509

(US8722683, 56)Show SMILES CO[C@H]1CC[C@H](CC(=O)N[C@H]2CC[C@H](CCN3CCN(CC3)c3cccc4OCOc34)CC2)CC1 |r,wU:10.9,2.1,wD:13.13,5.5,(11.55,4.52,;10.01,4.52,;9.24,3.19,;10.01,1.85,;9.24,.52,;7.7,.52,;6.93,-.82,;5.39,-.82,;4.62,-2.15,;4.62,.52,;3.08,.52,;2.31,-.82,;.77,-.82,;,.52,;-1.54,.52,;-2.31,-.82,;-3.85,-.82,;-4.62,-2.15,;-6.16,-2.15,;-6.93,-.82,;-6.16,.52,;-4.62,.52,;-8.47,-.82,;-9.24,.52,;-10.78,.52,;-11.55,-.82,;-10.78,-2.15,;-11.26,-3.61,;-10.01,-4.52,;-8.76,-3.61,;-9.24,-2.15,;.77,1.85,;2.31,1.85,;6.93,1.85,;7.7,3.19,)| Show InChI InChI=1S/C28H43N3O4/c1-33-24-11-7-22(8-12-24)19-27(32)29-23-9-5-21(6-10-23)13-14-30-15-17-31(18-16-30)25-3-2-4-26-28(25)35-20-34-26/h2-4,21-24H,5-20H2,1H3,(H,29,32)/t21-,22-,23-,24- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 3.37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121484

(US8722683, 31)Show SMILES O=C(C[C@H]1CCCCO1)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:10.10,3.2,wD:13.14,(5.39,-.67,;6.16,.67,;7.7,.67,;8.47,-.67,;10.01,-.67,;10.78,-2,;10.01,-3.33,;8.47,-3.33,;7.7,-2,;5.39,2,;3.85,2,;3.08,.67,;1.54,.67,;.77,2,;-.77,2,;-1.54,.67,;-3.08,.67,;-3.85,-.67,;-5.39,-.67,;-6.16,.67,;-5.39,2,;-3.85,2,;-7.7,.67,;-8.47,2,;-10.01,2,;-10.78,.67,;-10.01,-.67,;-10.49,-2.13,;-9.24,-3.04,;-7.99,-2.13,;-8.47,-.67,;1.54,3.33,;3.08,3.33,)| Show InChI InChI=1S/C26H39N3O4/c30-25(18-22-4-1-2-17-31-22)27-21-9-7-20(8-10-21)11-12-28-13-15-29(16-14-28)23-5-3-6-24-26(23)33-19-32-24/h3,5-6,20-22H,1-2,4,7-19H2,(H,27,30)/t20-,21-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 3.43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121468
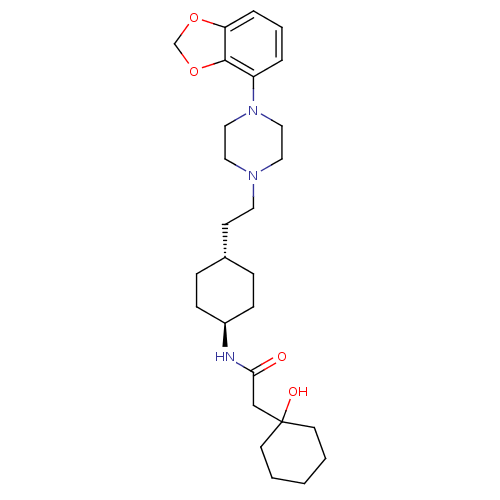
(US8722683, 15)Show SMILES OC1(CC(=O)N[C@H]2CC[C@H](CCN3CCN(CC3)c3cccc4OCOc34)CC2)CCCCC1 |r,wU:6.5,wD:9.9,(6.96,-1.59,;8.29,-.82,;7.52,.52,;5.98,.52,;5.21,-.82,;5.21,1.85,;3.67,1.85,;2.9,.52,;1.36,.52,;.59,1.85,;-.95,1.85,;-1.72,.52,;-3.26,.52,;-4.03,-.82,;-5.57,-.82,;-6.34,.52,;-5.57,1.85,;-4.03,1.85,;-7.88,.52,;-8.65,1.85,;-10.19,1.85,;-10.96,.52,;-10.19,-.82,;-10.66,-2.28,;-9.42,-3.19,;-8.17,-2.28,;-8.65,-.82,;1.36,3.19,;2.9,3.19,;8.29,-2.36,;9.63,-3.13,;10.96,-2.36,;10.96,-.82,;9.63,-.05,)| Show InChI InChI=1S/C27H41N3O4/c31-25(19-27(32)12-2-1-3-13-27)28-22-9-7-21(8-10-22)11-14-29-15-17-30(18-16-29)23-5-4-6-24-26(23)34-20-33-24/h4-6,21-22,32H,1-3,7-20H2,(H,28,31)/t21-,22- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 3.48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121483

(US8722683, 30)Show SMILES O=C(C[C@@H]1CCCCO1)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1 |r,wU:10.10,wD:13.14,3.2,(5.39,-.67,;6.16,.67,;7.7,.67,;8.47,-.67,;10.01,-.67,;10.78,-2,;10.01,-3.33,;8.47,-3.33,;7.7,-2,;5.39,2,;3.85,2,;3.08,.67,;1.54,.67,;.77,2,;-.77,2,;-1.54,.67,;-3.08,.67,;-3.85,-.67,;-5.39,-.67,;-6.16,.67,;-5.39,2,;-3.85,2,;-7.7,.67,;-8.47,2,;-10.01,2,;-10.78,.67,;-10.01,-.67,;-10.49,-2.13,;-9.24,-3.04,;-7.99,-2.13,;-8.47,-.67,;1.54,3.33,;3.08,3.33,)| Show InChI InChI=1S/C26H39N3O4/c30-25(18-22-4-1-2-17-31-22)27-21-9-7-20(8-10-21)11-12-28-13-15-29(16-14-28)23-5-3-6-24-26(23)33-19-32-24/h3,5-6,20-22H,1-2,4,7-19H2,(H,27,30)/t20-,21-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121512

(US8722683, 59)Show SMILES O=C(N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1)c1ccc(cc1)C#N |r,wU:3.2,wD:6.6,(3.85,-.82,;4.62,.52,;3.85,1.85,;2.31,1.85,;1.54,.52,;,.52,;-.77,1.85,;-2.31,1.85,;-3.08,.52,;-4.62,.52,;-5.39,-.82,;-6.93,-.82,;-7.7,.52,;-6.93,1.85,;-5.39,1.85,;-9.24,.52,;-10.01,1.85,;-11.55,1.85,;-12.32,.52,;-11.55,-.82,;-12.03,-2.28,;-10.78,-3.19,;-9.53,-2.28,;-10.01,-.82,;,3.19,;1.54,3.19,;6.16,.52,;6.93,1.85,;8.47,1.85,;9.24,.52,;8.47,-.82,;6.93,-.82,;10.78,.52,;12.32,.52,)| Show InChI InChI=1S/C27H32N4O3/c28-18-21-4-8-22(9-5-21)27(32)29-23-10-6-20(7-11-23)12-13-30-14-16-31(17-15-30)24-2-1-3-25-26(24)34-19-33-25/h1-5,8-9,20,23H,6-7,10-17,19H2,(H,29,32)/t20-,23- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 3.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM121505
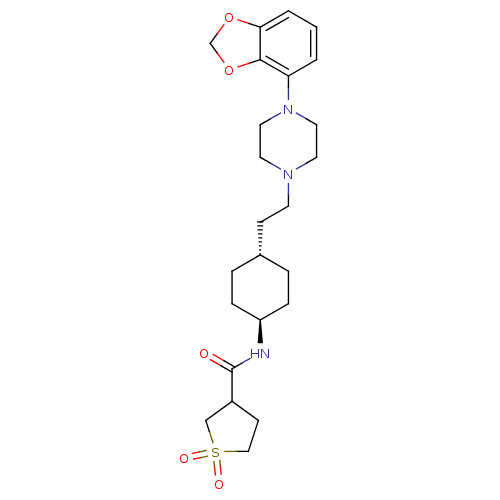
(US8722683, 52)Show SMILES O=C(N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1)C1CCS(=O)(=O)C1 |r,wU:3.2,wD:6.6,(4.98,-.82,;5.75,.52,;4.98,1.85,;3.44,1.85,;2.66,.52,;1.13,.52,;.36,1.85,;-1.18,1.85,;-1.95,.52,;-3.49,.52,;-4.26,-.82,;-5.8,-.82,;-6.57,.52,;-5.8,1.85,;-4.26,1.85,;-8.11,.52,;-8.88,1.85,;-10.42,1.85,;-11.19,.52,;-10.42,-.82,;-10.9,-2.28,;-9.65,-3.19,;-8.41,-2.28,;-8.88,-.82,;1.13,3.19,;2.66,3.19,;7.28,.52,;8.19,-.73,;9.65,-.25,;9.65,1.29,;11.19,1.29,;10.42,2.62,;8.19,1.76,)| Show InChI InChI=1S/C24H35N3O5S/c28-24(19-9-15-33(29,30)16-19)25-20-6-4-18(5-7-20)8-10-26-11-13-27(14-12-26)21-2-1-3-22-23(21)32-17-31-22/h1-3,18-20H,4-17H2,(H,25,28)/t18-,19?,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 3.56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann LA-Roche Inc.
US Patent
| Assay Description
Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl... |
US Patent US8722683 (2014)
BindingDB Entry DOI: 10.7270/Q2J101VJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































