

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin K (Homo sapiens (Human)) | BDBM123101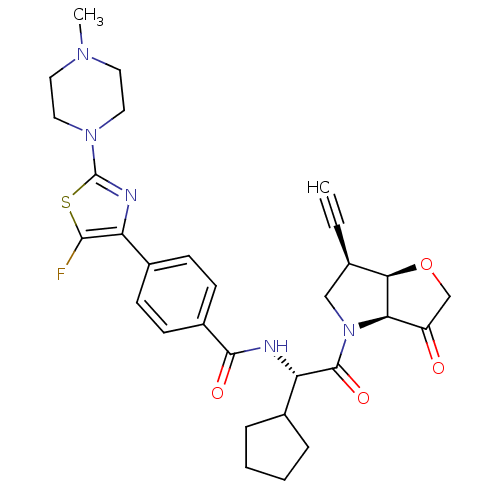 (US11312693, Example 5 | US8735395, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Medivir AB US Patent | Assay Description Standard assay conditions for the determination of kinetic constants used a fluorogenic peptide substrate, typically H-D-Ala-Leu-Lys-AMC, and were de... | US Patent US8735395 (2014) BindingDB Entry DOI: 10.7270/Q2QJ7G03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM123098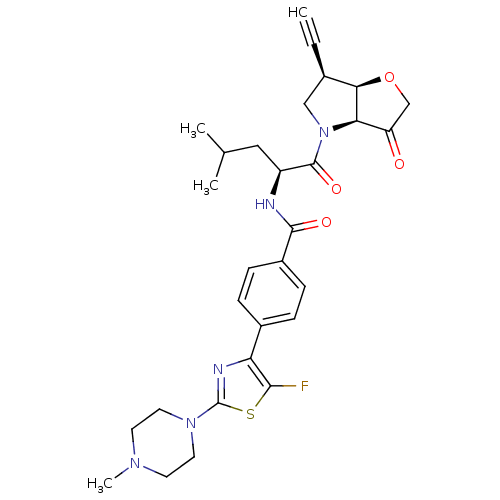 (US10723709, Example 2 | US11312693, Example 2 | US...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Medivir AB US Patent | Assay Description Standard assay conditions for the determination of kinetic constants used a fluorogenic peptide substrate, typically H-D-Ala-Leu-Lys-AMC, and were de... | US Patent US8735395 (2014) BindingDB Entry DOI: 10.7270/Q2QJ7G03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM123097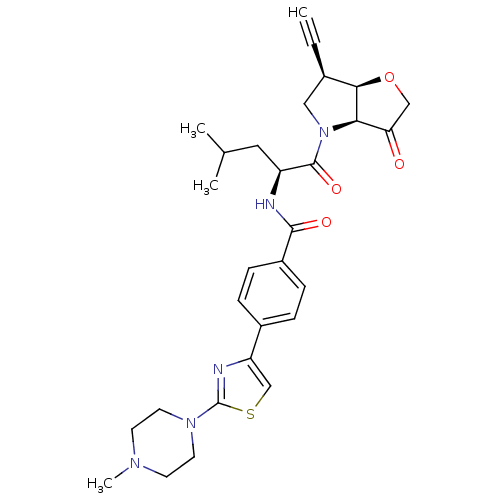 (US10723709, Example 1 | US11312693, Example 1 | US...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Medivir AB US Patent | Assay Description Standard assay conditions for the determination of kinetic constants used a fluorogenic peptide substrate, typically H-D-Ala-Leu-Lys-AMC, and were de... | US Patent US8735395 (2014) BindingDB Entry DOI: 10.7270/Q2QJ7G03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM123100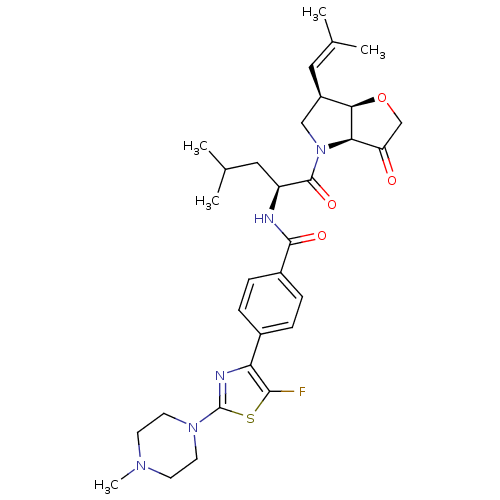 (US10723709, Example 4 | US11312693, Example 4 | US...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Medivir AB US Patent | Assay Description Standard assay conditions for the determination of kinetic constants used a fluorogenic peptide substrate, typically H-D-Ala-Leu-Lys-AMC, and were de... | US Patent US8735395 (2014) BindingDB Entry DOI: 10.7270/Q2QJ7G03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM123099 (US10723709, Example 3 | US11312693, Example 3 | US...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Medivir AB US Patent | Assay Description Standard assay conditions for the determination of kinetic constants used a fluorogenic peptide substrate, typically H-D-Ala-Leu-Lys-AMC, and were de... | US Patent US8735395 (2014) BindingDB Entry DOI: 10.7270/Q2QJ7G03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM123100 (US10723709, Example 4 | US11312693, Example 4 | US...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB US Patent | Assay Description The enzyme is commercially available human cathepsin L (for example Calbiochem). The substrate is H-D-Val-Leu-Lys-AMC available from Bahcem. The assa... | US Patent US8735395 (2014) BindingDB Entry DOI: 10.7270/Q2QJ7G03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM123099 (US10723709, Example 3 | US11312693, Example 3 | US...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB US Patent | Assay Description The enzyme is commercially available human cathepsin L (for example Calbiochem). The substrate is H-D-Val-Leu-Lys-AMC available from Bahcem. The assa... | US Patent US8735395 (2014) BindingDB Entry DOI: 10.7270/Q2QJ7G03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM123098 (US10723709, Example 2 | US11312693, Example 2 | US...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB US Patent | Assay Description The enzyme is commercially available human cathepsin L (for example Calbiochem). The substrate is H-D-Val-Leu-Lys-AMC available from Bahcem. The assa... | US Patent US8735395 (2014) BindingDB Entry DOI: 10.7270/Q2QJ7G03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM123097 (US10723709, Example 1 | US11312693, Example 1 | US...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB US Patent | Assay Description The enzyme is commercially available human cathepsin L (for example Calbiochem). The substrate is H-D-Val-Leu-Lys-AMC available from Bahcem. The assa... | US Patent US8735395 (2014) BindingDB Entry DOI: 10.7270/Q2QJ7G03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM123101 (US11312693, Example 5 | US8735395, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB US Patent | Assay Description The assay uses baculovirus-expressed human cathepsin S and the boc-Val-Leu-Lys-AMC fluorescent substrate available from Bachem in a 384 well plate fo... | US Patent US8735395 (2014) BindingDB Entry DOI: 10.7270/Q2QJ7G03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM123100 (US10723709, Example 4 | US11312693, Example 4 | US...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB US Patent | Assay Description The assay uses baculovirus-expressed human cathepsin S and the boc-Val-Leu-Lys-AMC fluorescent substrate available from Bachem in a 384 well plate fo... | US Patent US8735395 (2014) BindingDB Entry DOI: 10.7270/Q2QJ7G03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM123098 (US10723709, Example 2 | US11312693, Example 2 | US...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB US Patent | Assay Description The assay uses baculovirus-expressed human cathepsin S and the boc-Val-Leu-Lys-AMC fluorescent substrate available from Bachem in a 384 well plate fo... | US Patent US8735395 (2014) BindingDB Entry DOI: 10.7270/Q2QJ7G03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM123097 (US10723709, Example 1 | US11312693, Example 1 | US...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB US Patent | Assay Description The assay uses baculovirus-expressed human cathepsin S and the boc-Val-Leu-Lys-AMC fluorescent substrate available from Bachem in a 384 well plate fo... | US Patent US8735395 (2014) BindingDB Entry DOI: 10.7270/Q2QJ7G03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||