

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM136702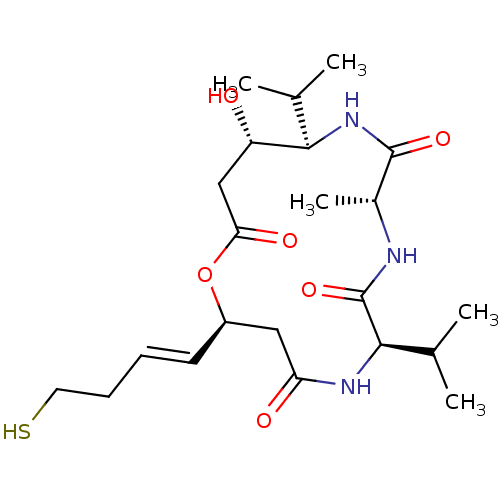 (US8865655, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | 25 |
Karus Therapeutics Limited US Patent | Assay Description In vitro HDAC assays were performed using a HDAC fluorescent activity assay kit (Biomol, UK) according to the manufacturer's instructions. Compounds ... | US Patent US8865655 (2014) BindingDB Entry DOI: 10.7270/Q2Z036V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM136703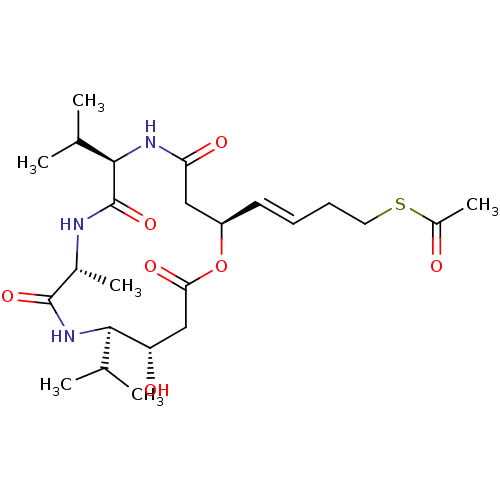 (US8865655, 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 21.6 | n/a | n/a | n/a | n/a | n/a | 25 |
Karus Therapeutics Limited US Patent | Assay Description In vitro HDAC assays were performed using a HDAC fluorescent activity assay kit (Biomol, UK) according to the manufacturer's instructions. Compounds ... | US Patent US8865655 (2014) BindingDB Entry DOI: 10.7270/Q2Z036V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM136704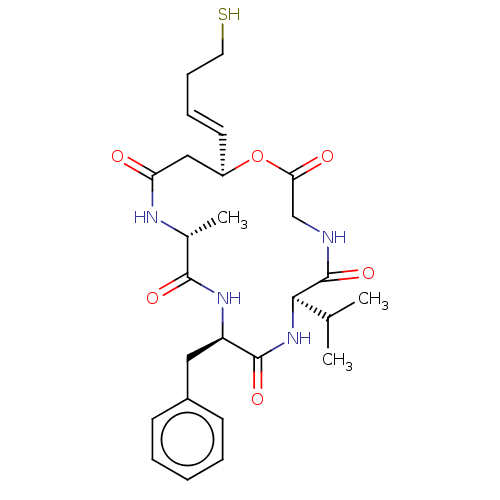 (US8865655, 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | 25 |
Karus Therapeutics Limited US Patent | Assay Description In vitro HDAC assays were performed using a HDAC fluorescent activity assay kit (Biomol, UK) according to the manufacturer's instructions. Compounds ... | US Patent US8865655 (2014) BindingDB Entry DOI: 10.7270/Q2Z036V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM136705 (US8865655, 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | 25 |
Karus Therapeutics Limited US Patent | Assay Description In vitro HDAC assays were performed using a HDAC fluorescent activity assay kit (Biomol, UK) according to the manufacturer's instructions. Compounds ... | US Patent US8865655 (2014) BindingDB Entry DOI: 10.7270/Q2Z036V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||