

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138764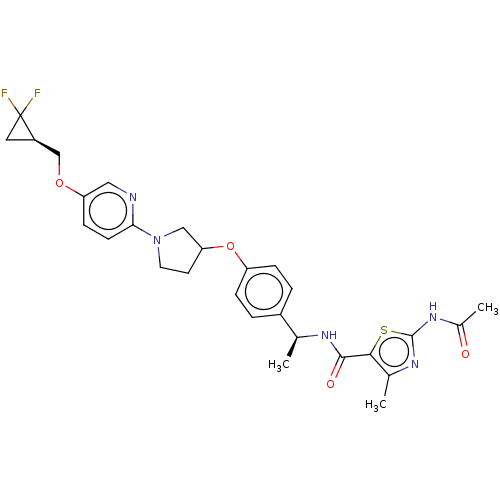 (US8877741, 2.90) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138632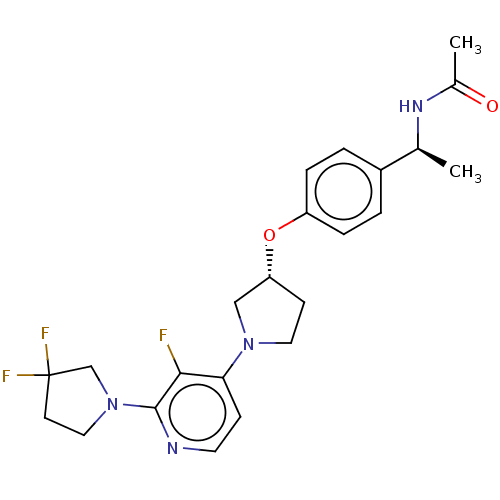 (US8877741, 1.110) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138626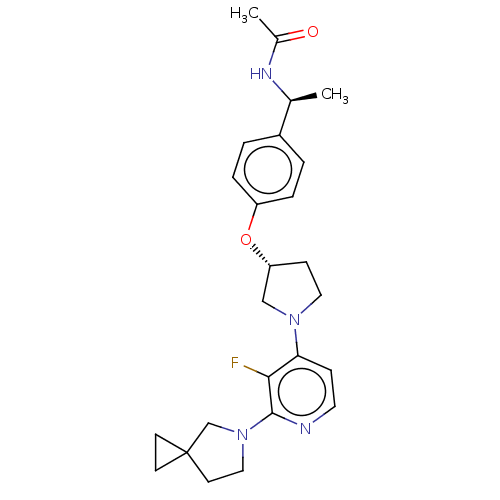 (US8877741, 1.104) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138743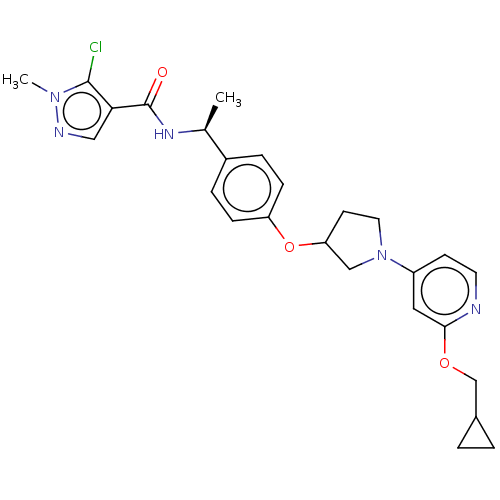 (US8877741, 2.69 | US8877741, 2.76) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138674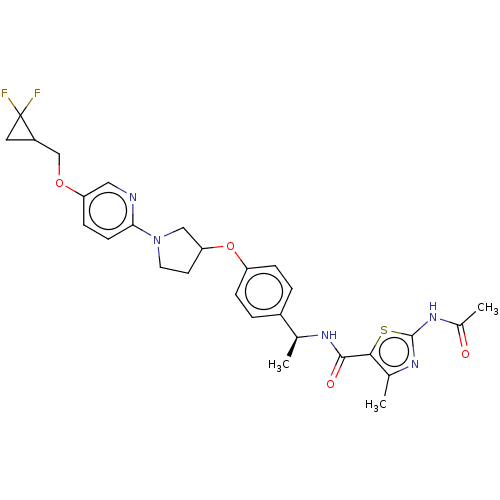 (US8877741, 2.1 | US8877741, 524) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 25 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138660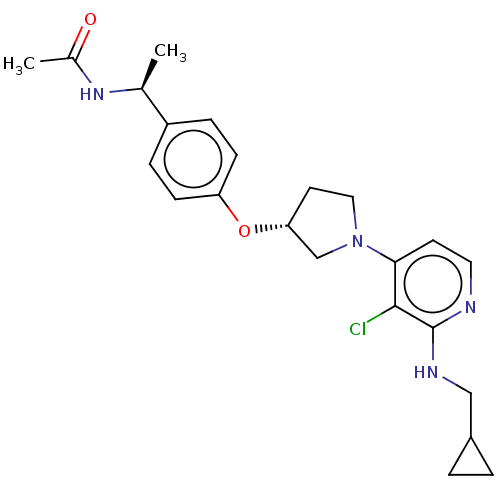 (US8877741, 1.138) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138599 (US8877741, 1.77) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 29 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138597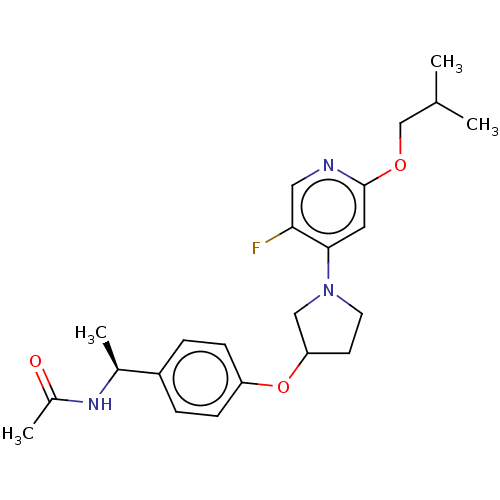 (US8877741, 1.75) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138893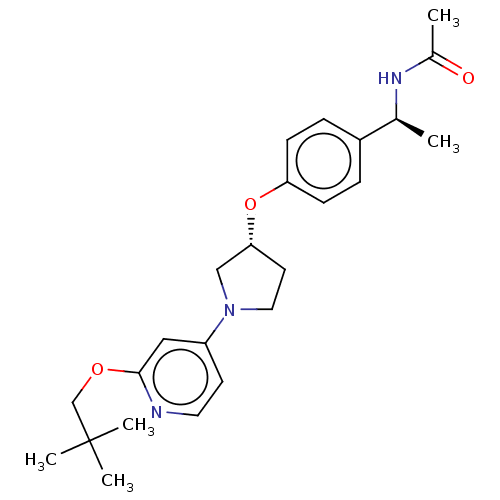 (US8877741, 9.29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138649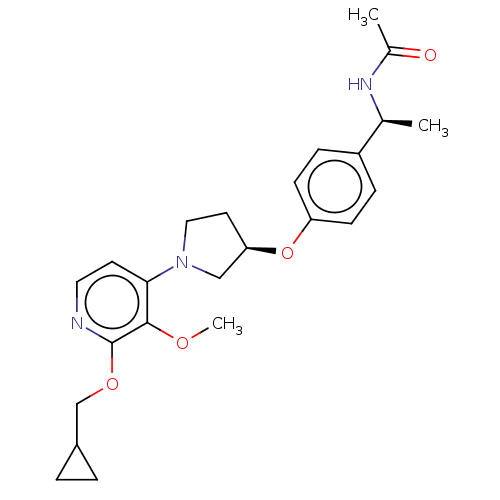 (US8877741, 1.127) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 31 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138642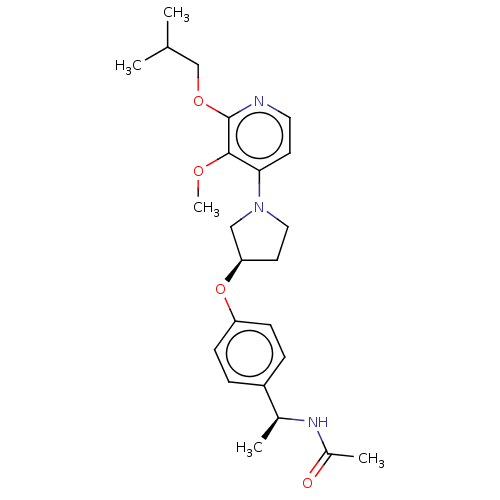 (US8877741, 1.120) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 32 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138838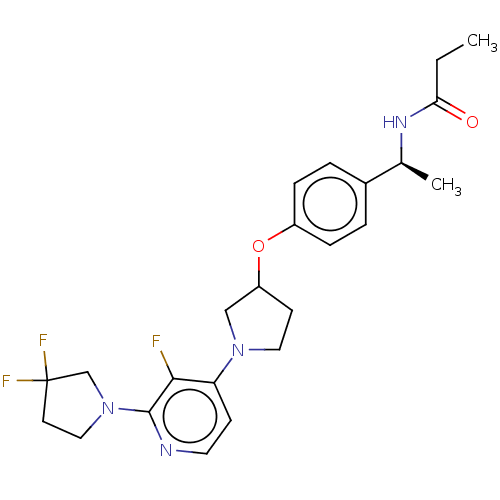 (US8877741, 5.3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 34 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138928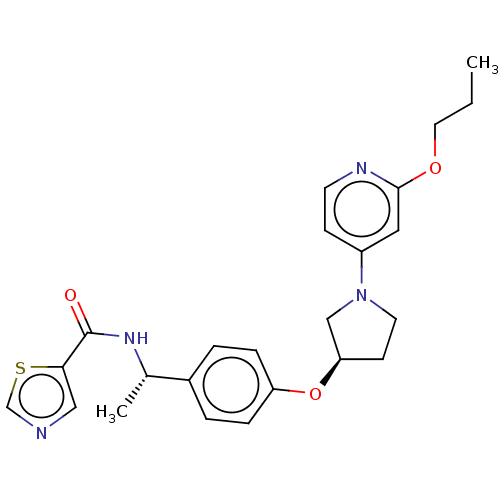 (US8877741, 9.64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138734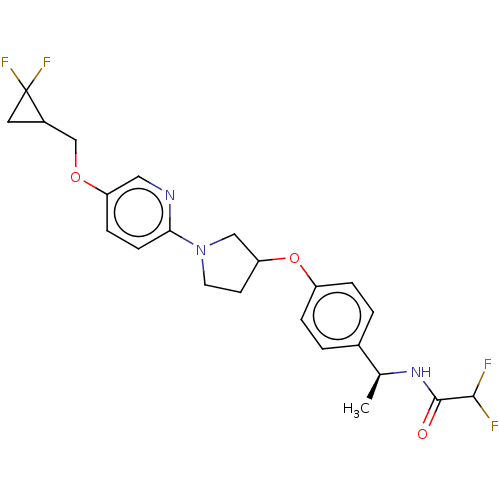 (US8877741, 2.60) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138969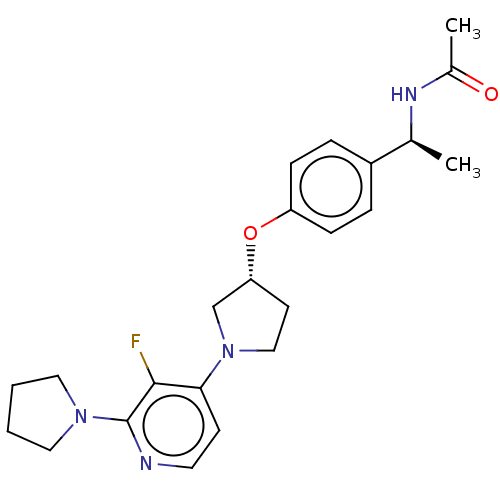 (US8877741, 10.16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138755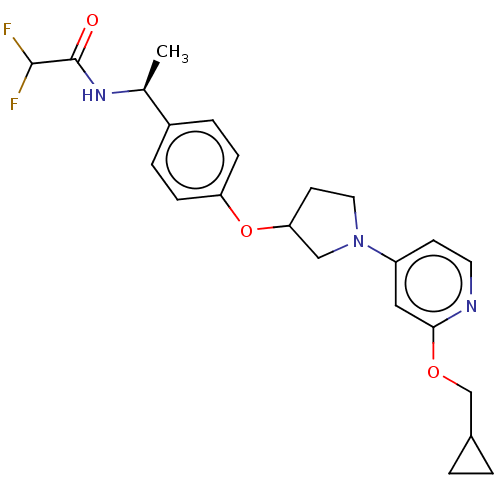 (US8877741, 2.81) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138946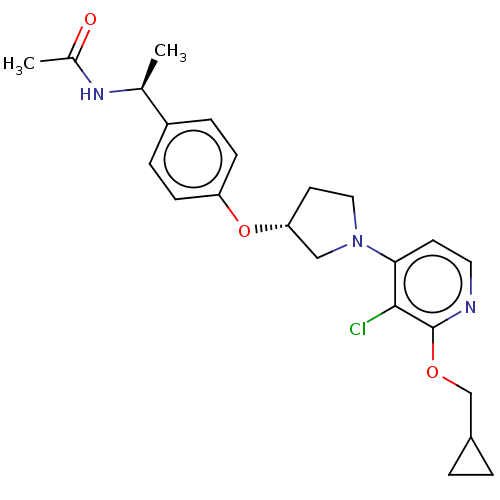 (US8877741, 9.82) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138735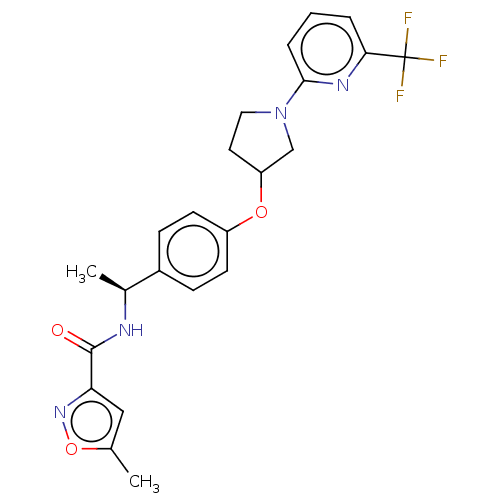 (US8877741, 2.61) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138972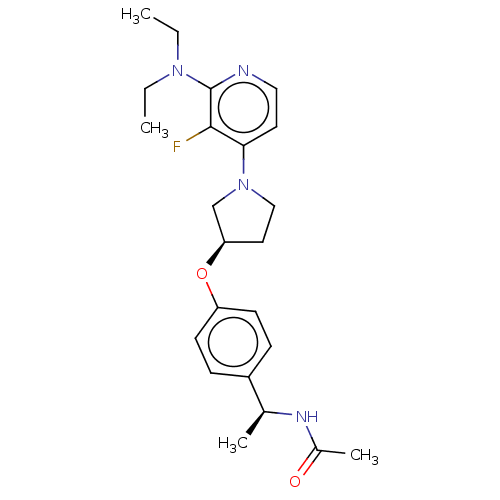 (US8877741, 10.19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138880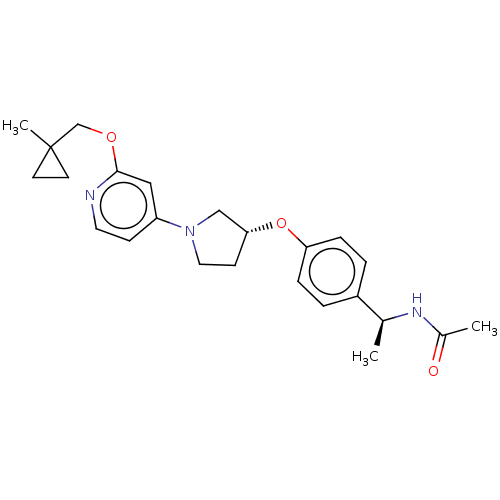 (US8877741, 9.16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138841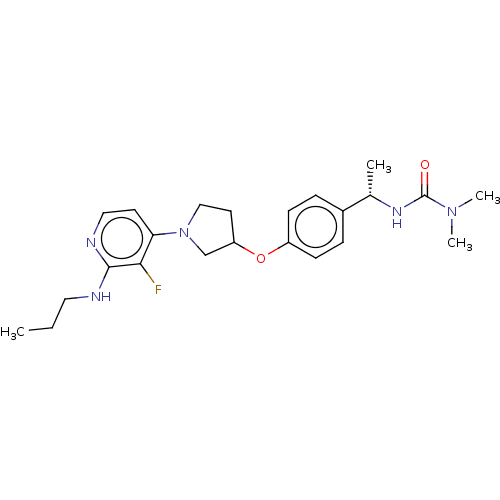 (US8877741, 5.6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 36 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138661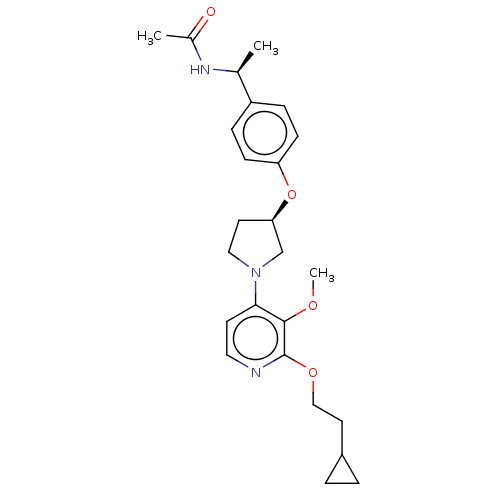 (US8877741, 1.139) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 37 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138794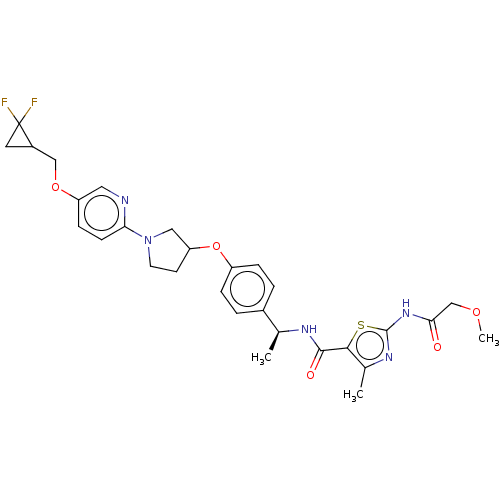 (US8877741, 2.120) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 37 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138864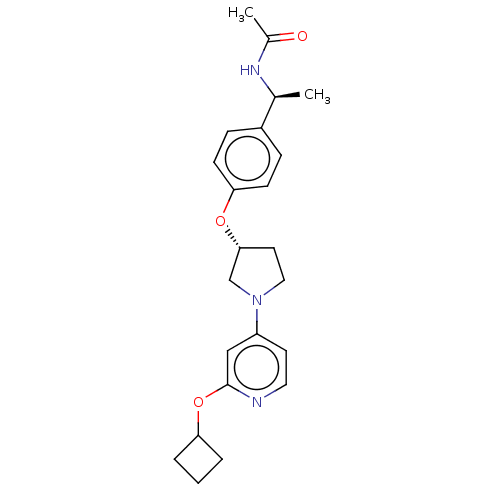 (US8877741, 714 | US8877741, 9.1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 39 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138749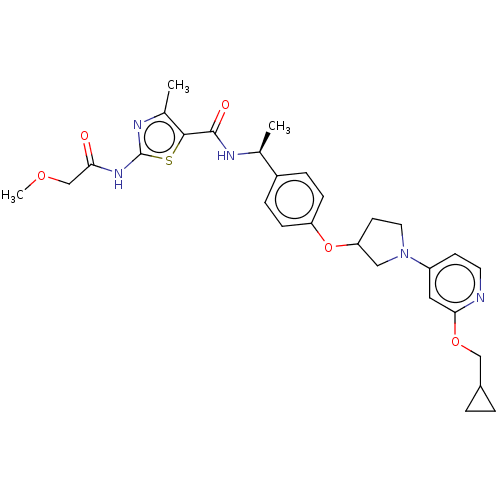 (US8877741, 2.75) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 39 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138825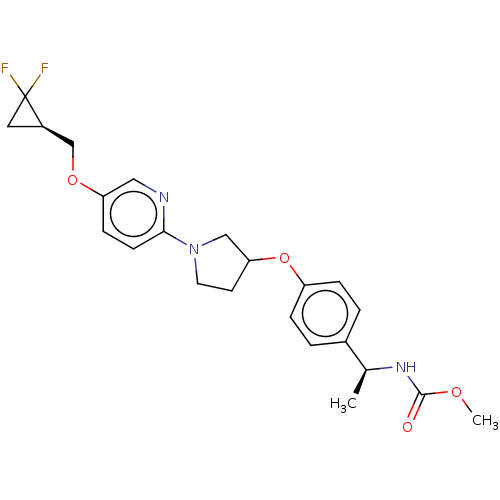 (US8877741, 4.1 | US8877741, 675) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 39 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138640 (US8877741, 1.118) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 39 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138892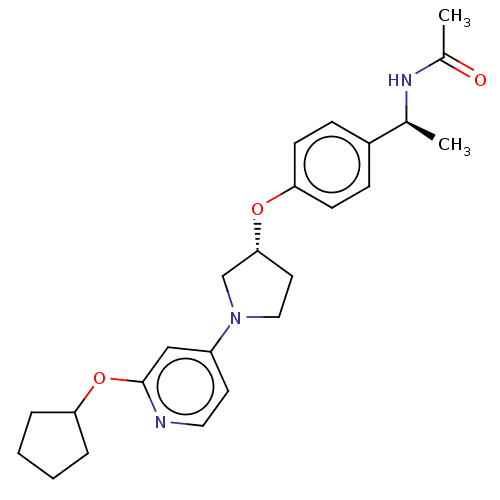 (US8877741, 9.28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138917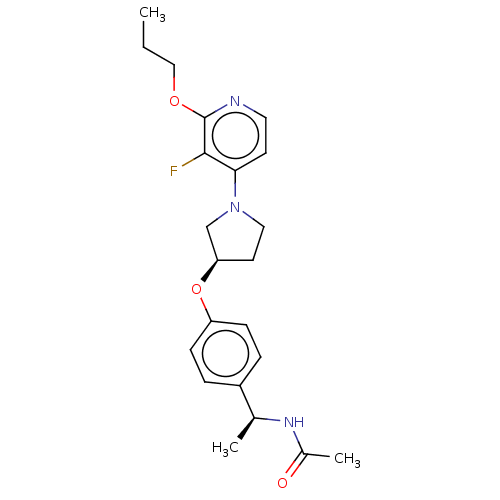 (US8877741, 9.53) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138871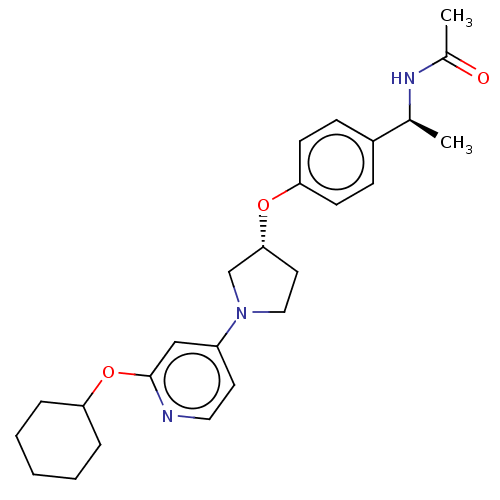 (US8877741, 9.7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138767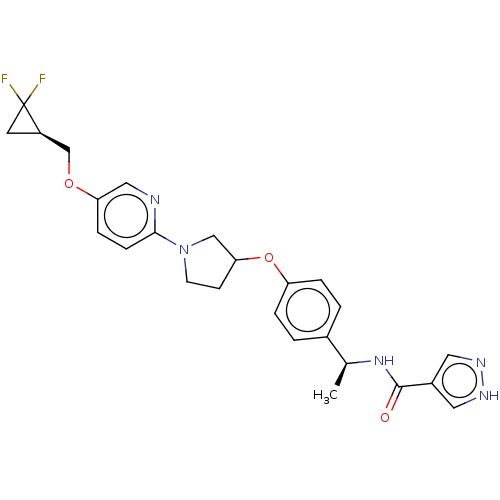 (US8877741, 2.93) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138924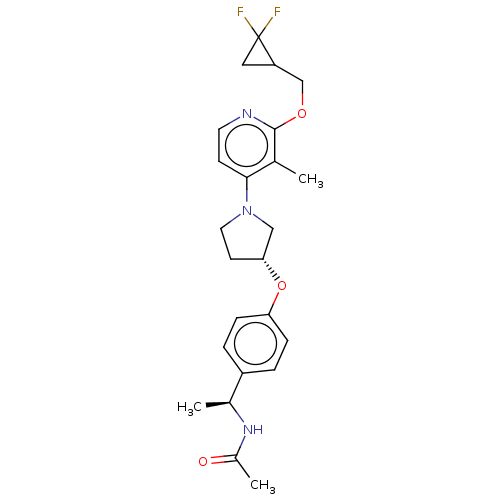 (US8877741, 9.60) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138879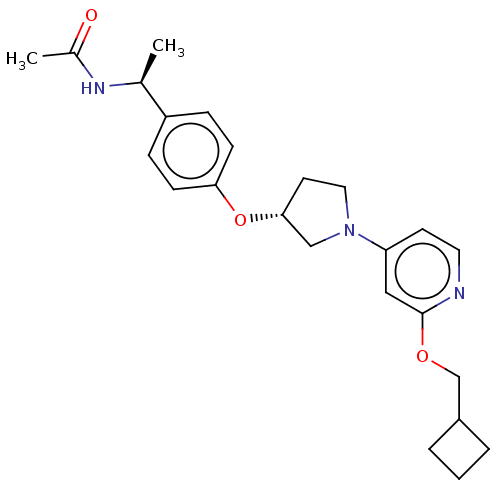 (US8877741, 9.15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138866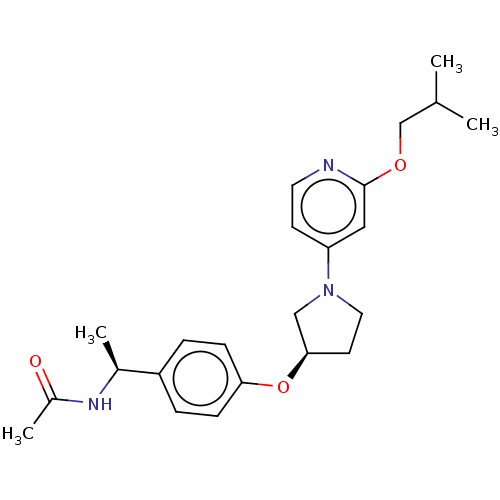 (US8877741, 9.2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 41 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138643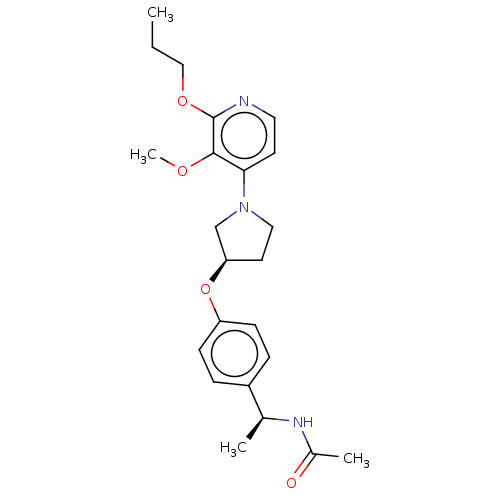 (US8877741, 1.121) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 41 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138654 (US8877741, 1.132) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 41 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138630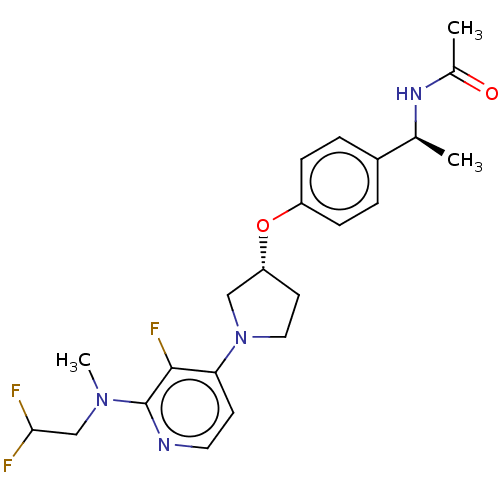 (US8877741, 1.108) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 42 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138915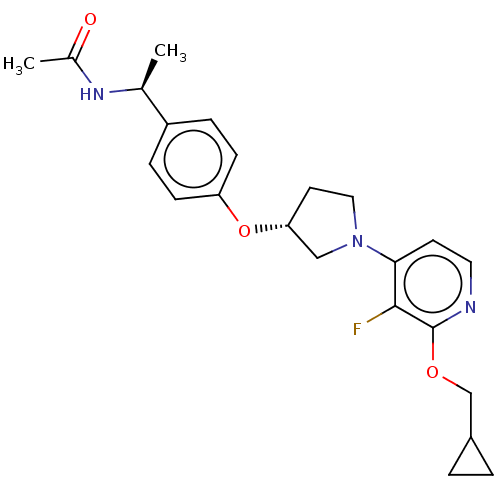 (US8877741, 9.51) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 42 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138795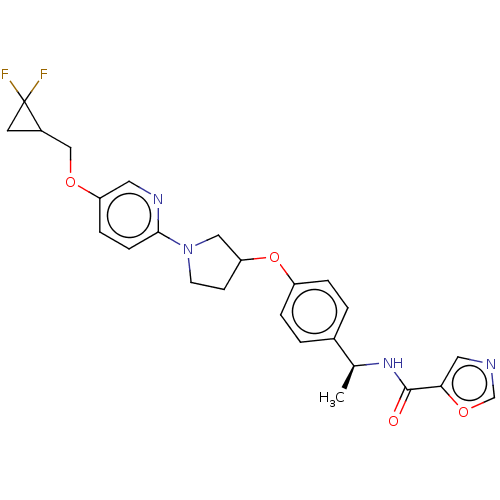 (US8877741, 2.121) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 43 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138636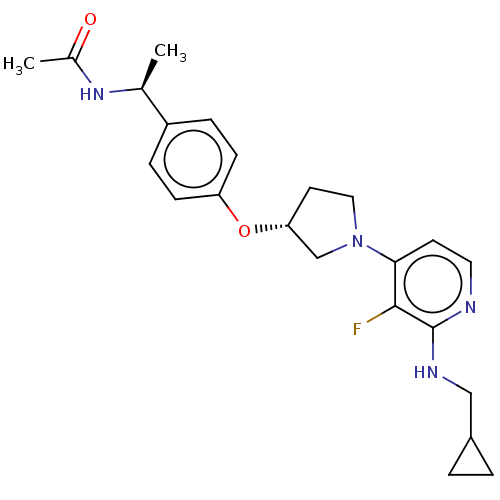 (US8877741, 1.114 | US8877741, 1.147) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 43 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138926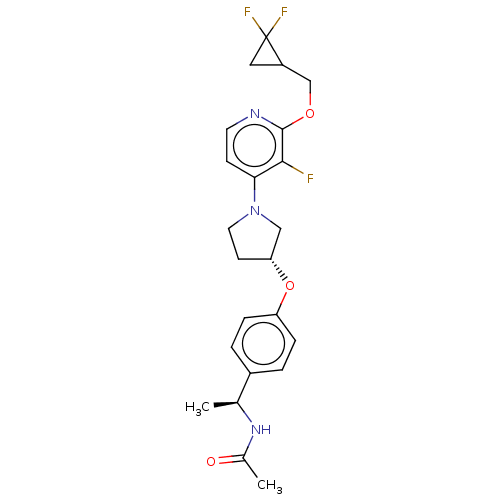 (US8877741, 9.62) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 44 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM139017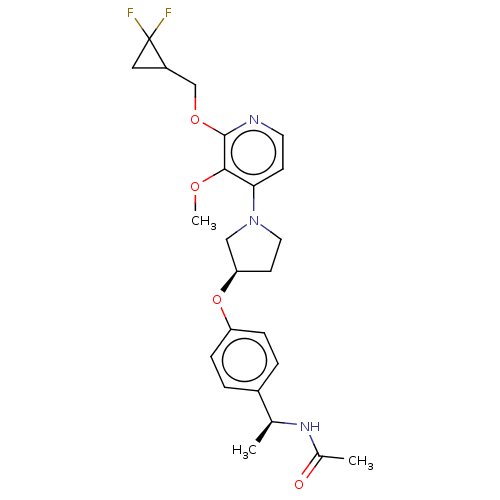 (US8877741, 14.2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 45 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138887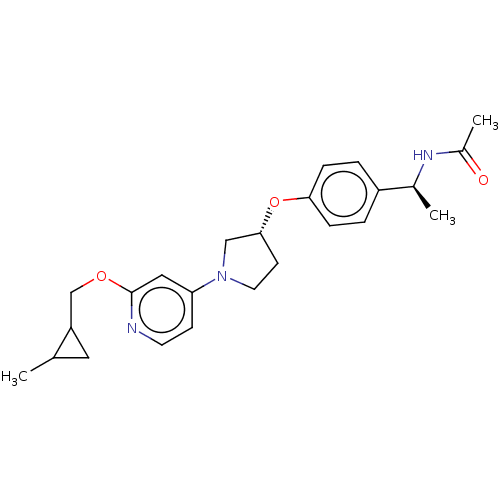 (US8877741, 9.23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 45 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138937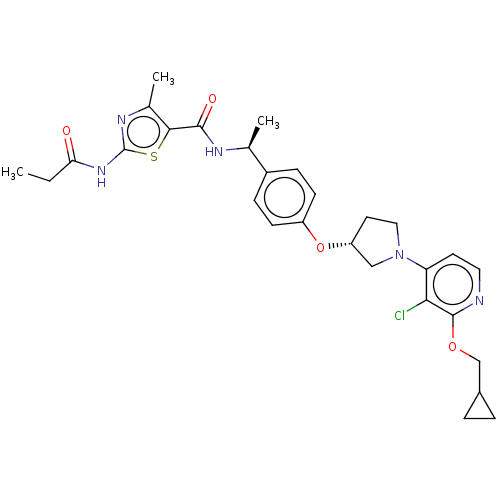 (US8877741, 9.73) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 45 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138935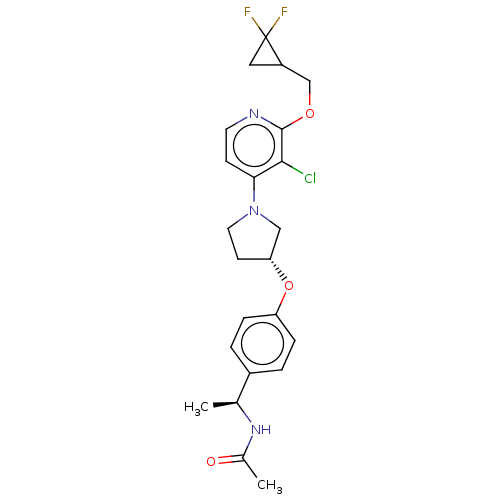 (US8877741, 9.71) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 45 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138948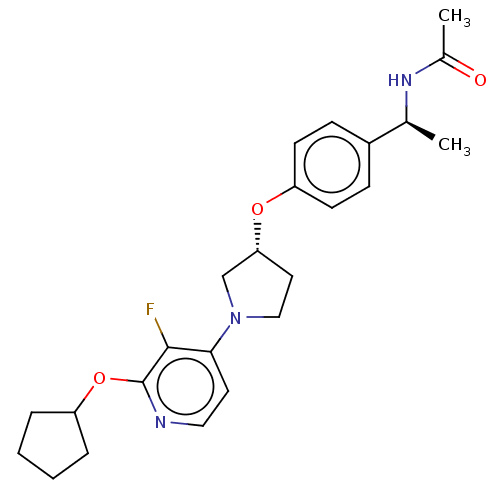 (US8877741, 9.84) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 45 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138967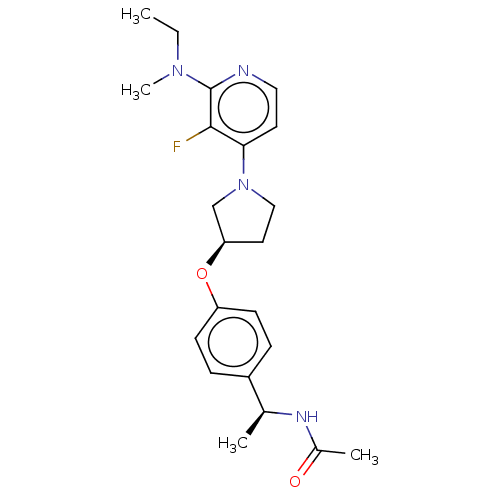 (US8877741, 10.14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 45 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138800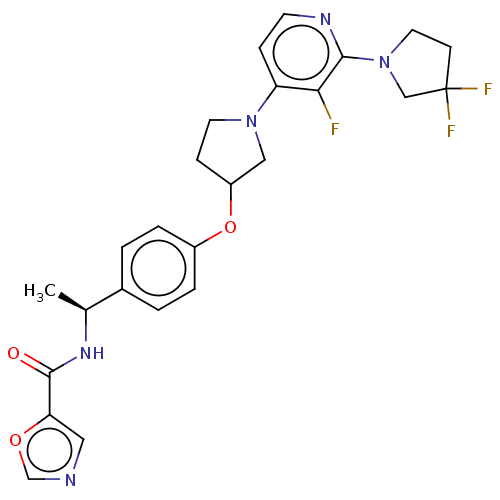 (US8877741, 2.126) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 45 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138738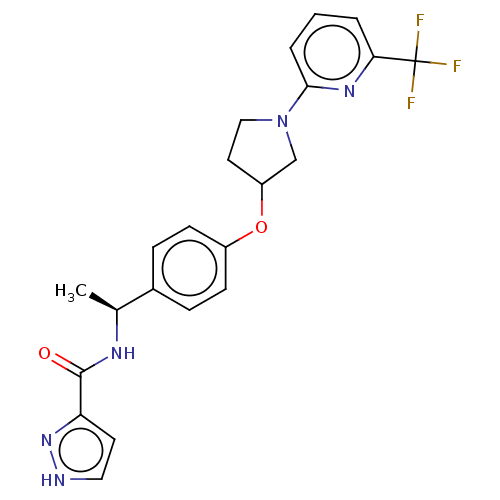 (US8877741, 2.64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 45 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM138523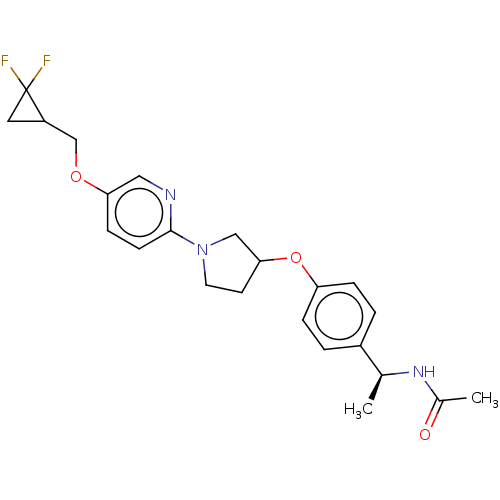 (US8877741, 1.1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 46 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description Malonyl CoA formation by acetyl CoA carboxylases is stoichometrically linked to the consumption of ATP. ACC2 activity is measured in a NADH-linked ki... | US Patent US8877741 (2014) BindingDB Entry DOI: 10.7270/Q2R78CXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 485 total ) | Next | Last >> |