

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM12341 (CHEMBL178681 | CHEMBL359657 | US8609708, 1 | US860...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 160 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA US Patent | Assay Description To gain insight into the selectivity of the synthetic compounds for inhibition of other CYPs, we examined the major CYPs present in human liver. Prio... | US Patent US8906943 (2014) BindingDB Entry DOI: 10.7270/Q23F4NBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM50188097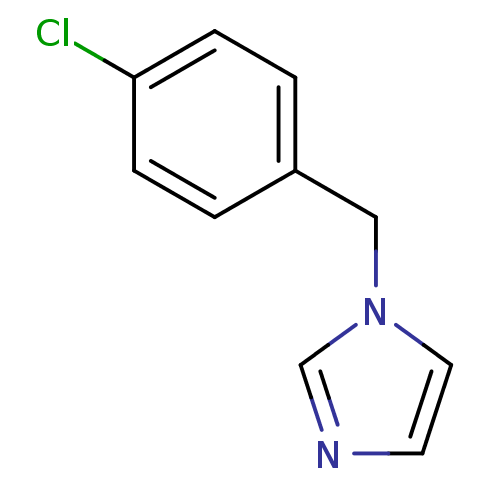 (1-(4-Chlorobenzyl)-1H-imidazole | CHEMBL441367) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 160 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA US Patent | Assay Description To gain insight into the selectivity of the synthetic compounds for inhibition of other CYPs, we examined the major CYPs present in human liver. Prio... | US Patent US8906943 (2014) BindingDB Entry DOI: 10.7270/Q23F4NBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM12351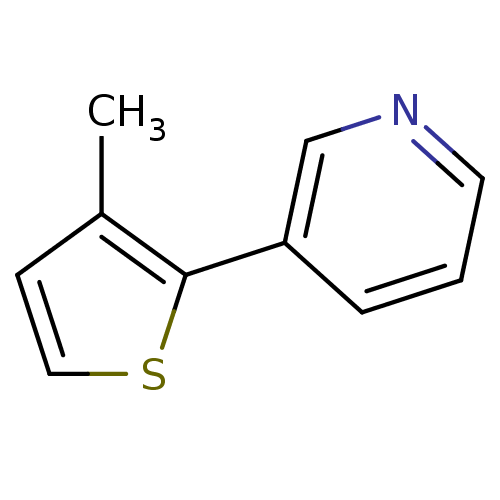 (3-(3-methylthiophen-2-yl)pyridine | CHEMBL179669 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA US Patent | Assay Description To gain insight into the selectivity of the synthetic compounds for inhibition of other CYPs, we examined the major CYPs present in human liver. Prio... | US Patent US8906943 (2014) BindingDB Entry DOI: 10.7270/Q23F4NBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM12345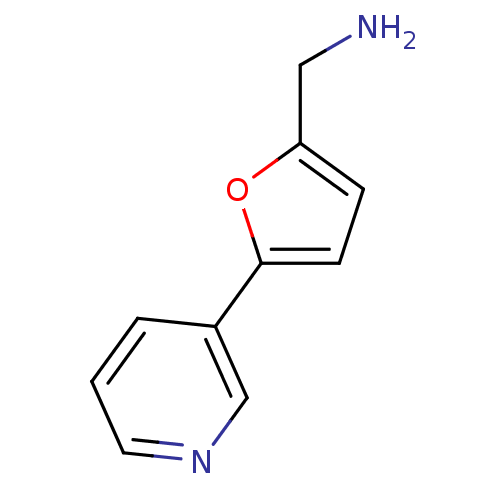 (CHEMBL178090 | US8609708, 2 | US8609708, 47 | [5-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA US Patent | Assay Description To gain insight into the selectivity of the synthetic compounds for inhibition of other CYPs, we examined the major CYPs present in human liver. Prio... | US Patent US8906943 (2014) BindingDB Entry DOI: 10.7270/Q23F4NBW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM12370 ((3-(Pyridin-3-yl)-1H-pyrazol-5-yl)methanamine | [3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 600 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA US Patent | Assay Description To gain insight into the selectivity of the synthetic compounds for inhibition of other CYPs, we examined the major CYPs present in human liver. Prio... | US Patent US8906943 (2014) BindingDB Entry DOI: 10.7270/Q23F4NBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM12371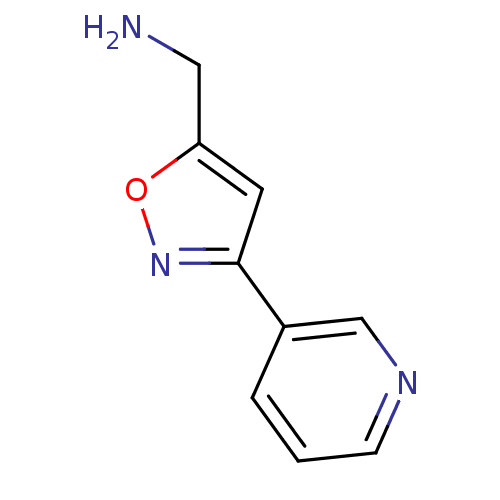 ((3-(Pyridin-3-yl)isoxazol-5-yl)methanamine Dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 800 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA US Patent | Assay Description To gain insight into the selectivity of the synthetic compounds for inhibition of other CYPs, we examined the major CYPs present in human liver. Prio... | US Patent US8906943 (2014) BindingDB Entry DOI: 10.7270/Q23F4NBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM12351 (3-(3-methylthiophen-2-yl)pyridine | CHEMBL179669 |...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA US Patent | Assay Description To gain insight into the selectivity of the synthetic compounds for inhibition of other CYPs, we examined the major CYPs present in human liver. Prio... | US Patent US8906943 (2014) BindingDB Entry DOI: 10.7270/Q23F4NBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM50149352 (1-Cyclohexylmethyl-1H-imidazole | CHEMBL120057) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA US Patent | Assay Description To gain insight into the selectivity of the synthetic compounds for inhibition of other CYPs, we examined the major CYPs present in human liver. Prio... | US Patent US8906943 (2014) BindingDB Entry DOI: 10.7270/Q23F4NBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM50188097 (1-(4-Chlorobenzyl)-1H-imidazole | CHEMBL441367) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA US Patent | Assay Description To gain insight into the selectivity of the synthetic compounds for inhibition of other CYPs, we examined the major CYPs present in human liver. Prio... | US Patent US8906943 (2014) BindingDB Entry DOI: 10.7270/Q23F4NBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM140266 (US8906943, 11) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA US Patent | Assay Description To gain insight into the selectivity of the synthetic compounds for inhibition of other CYPs, we examined the major CYPs present in human liver. Prio... | US Patent US8906943 (2014) BindingDB Entry DOI: 10.7270/Q23F4NBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM12341 (CHEMBL178681 | CHEMBL359657 | US8609708, 1 | US860...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 5.22E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA US Patent | Assay Description To gain insight into the selectivity of the synthetic compounds for inhibition of other CYPs, we examined the major CYPs present in human liver. Prio... | US Patent US8906943 (2014) BindingDB Entry DOI: 10.7270/Q23F4NBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM12370 ((3-(Pyridin-3-yl)-1H-pyrazol-5-yl)methanamine | [3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 6.64E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA US Patent | Assay Description To gain insight into the selectivity of the synthetic compounds for inhibition of other CYPs, we examined the major CYPs present in human liver. Prio... | US Patent US8906943 (2014) BindingDB Entry DOI: 10.7270/Q23F4NBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM12371 ((3-(Pyridin-3-yl)isoxazol-5-yl)methanamine Dihydro...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.74E+4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA US Patent | Assay Description To gain insight into the selectivity of the synthetic compounds for inhibition of other CYPs, we examined the major CYPs present in human liver. Prio... | US Patent US8906943 (2014) BindingDB Entry DOI: 10.7270/Q23F4NBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM12357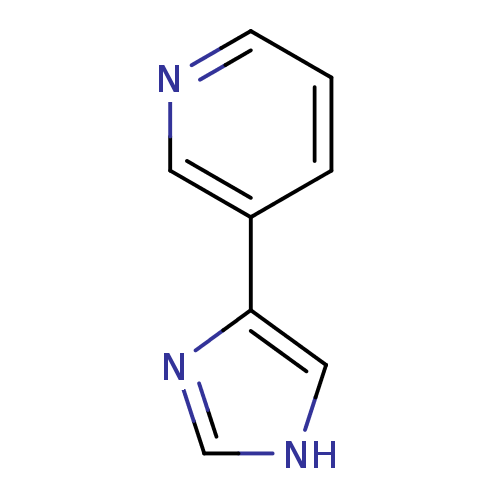 (3-(1H-imidazol-4-yl)pyridine | CHEMBL178516 | JMC5...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.03E+5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA US Patent | Assay Description To gain insight into the selectivity of the synthetic compounds for inhibition of other CYPs, we examined the major CYPs present in human liver. Prio... | US Patent US8906943 (2014) BindingDB Entry DOI: 10.7270/Q23F4NBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM50149352 (1-Cyclohexylmethyl-1H-imidazole | CHEMBL120057) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 1.70E+5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA US Patent | Assay Description To gain insight into the selectivity of the synthetic compounds for inhibition of other CYPs, we examined the major CYPs present in human liver. Prio... | US Patent US8906943 (2014) BindingDB Entry DOI: 10.7270/Q23F4NBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM12345 (CHEMBL178090 | US8609708, 2 | US8609708, 47 | [5-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 1.91E+5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA US Patent | Assay Description To gain insight into the selectivity of the synthetic compounds for inhibition of other CYPs, we examined the major CYPs present in human liver. Prio... | US Patent US8906943 (2014) BindingDB Entry DOI: 10.7270/Q23F4NBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM45885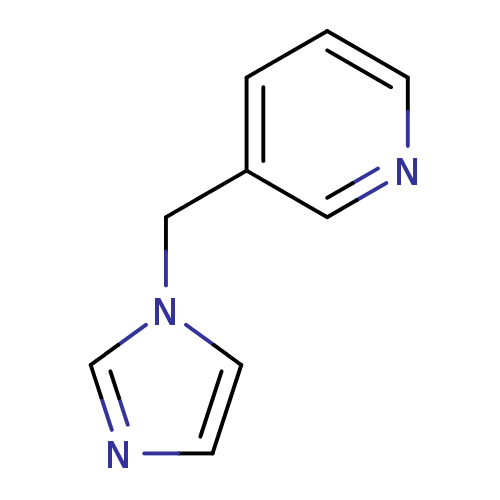 (3-(1-imidazolylmethyl)pyridine | 3-(imidazol-1-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA US Patent | Assay Description To gain insight into the selectivity of the synthetic compounds for inhibition of other CYPs, we examined the major CYPs present in human liver. Prio... | US Patent US8906943 (2014) BindingDB Entry DOI: 10.7270/Q23F4NBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM140266 (US8906943, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.18E+5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA US Patent | Assay Description To gain insight into the selectivity of the synthetic compounds for inhibition of other CYPs, we examined the major CYPs present in human liver. Prio... | US Patent US8906943 (2014) BindingDB Entry DOI: 10.7270/Q23F4NBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2A6 (Homo sapiens (Human)) | BDBM12357 (3-(1H-imidazol-4-yl)pyridine | CHEMBL178516 | JMC5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 8.00E+5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TBA US Patent | Assay Description To gain insight into the selectivity of the synthetic compounds for inhibition of other CYPs, we examined the major CYPs present in human liver. Prio... | US Patent US8906943 (2014) BindingDB Entry DOI: 10.7270/Q23F4NBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||