

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50334454 (CHEMBL1643895 | Ramosetron | US9045501, Ramosetron) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | 0.0600 | -58.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinity of the various compounds for the human 5-HT3 receptor was measured in a radioligand binding assay, using a scintillation proxim... | US Patent US9045501 (2015) BindingDB Entry DOI: 10.7270/Q2MK6BN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50014549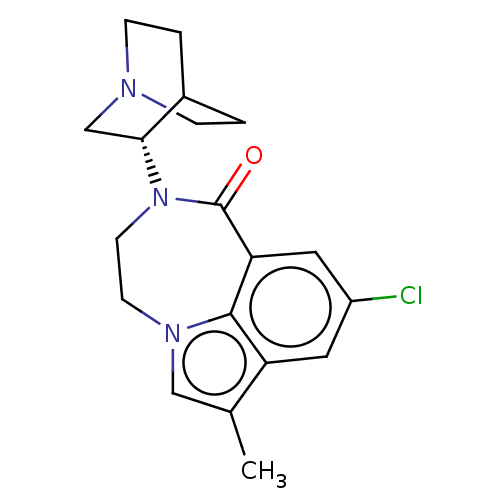 (CHEMBL3261480 | US9045501, 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.200 | -55.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinity of the various compounds for the human 5-HT3 receptor was measured in a radioligand binding assay, using a scintillation proxim... | US Patent US9045501 (2015) BindingDB Entry DOI: 10.7270/Q2MK6BN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50014558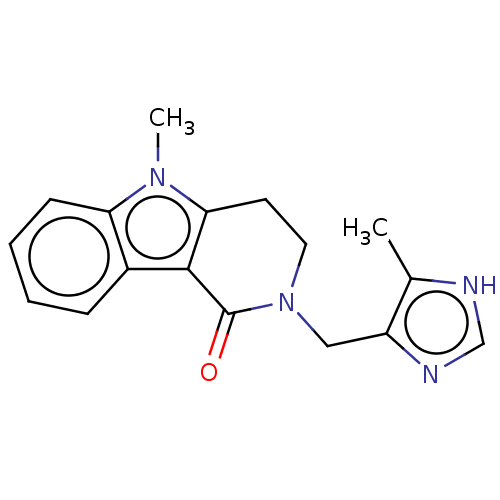 (ALOSETRON | CHEBI:253342 | Lotronex | US9045501, A...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank US Patent | 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinity of the various compounds for the human 5-HT3 receptor was measured in a radioligand binding assay, using a scintillation proxim... | US Patent US9045501 (2015) BindingDB Entry DOI: 10.7270/Q2MK6BN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50014552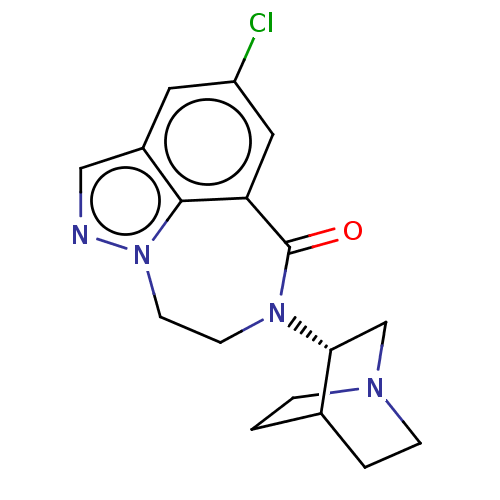 (CHEMBL3261483 | US9045501, 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinity of the various compounds for the human 5-HT3 receptor was measured in a radioligand binding assay, using a scintillation proxim... | US Patent US9045501 (2015) BindingDB Entry DOI: 10.7270/Q2MK6BN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM160716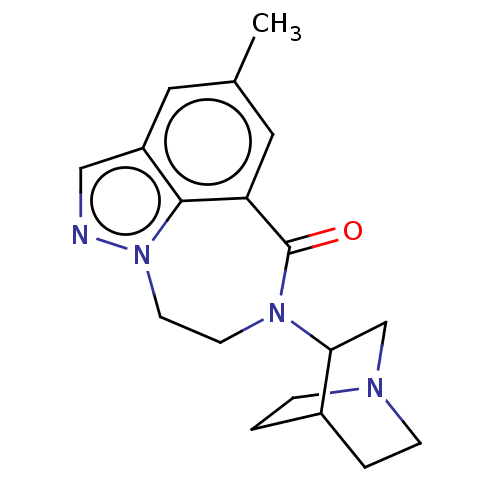 (US9045501, 11) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.800 | -51.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinity of the various compounds for the human 5-HT3 receptor was measured in a radioligand binding assay, using a scintillation proxim... | US Patent US9045501 (2015) BindingDB Entry DOI: 10.7270/Q2MK6BN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM160717 (US9045501, 12) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinity of the various compounds for the human 5-HT3 receptor was measured in a radioligand binding assay, using a scintillation proxim... | US Patent US9045501 (2015) BindingDB Entry DOI: 10.7270/Q2MK6BN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM160732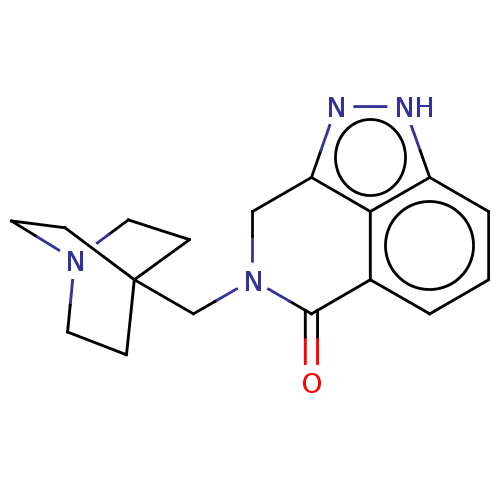 (US9045501, 29) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinity of the various compounds for the human 5-HT3 receptor was measured in a radioligand binding assay, using a scintillation proxim... | US Patent US9045501 (2015) BindingDB Entry DOI: 10.7270/Q2MK6BN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50014548 (CHEMBL3261479 | US9045501, 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 4 | -47.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinity of the various compounds for the human 5-HT3 receptor was measured in a radioligand binding assay, using a scintillation proxim... | US Patent US9045501 (2015) BindingDB Entry DOI: 10.7270/Q2MK6BN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM160711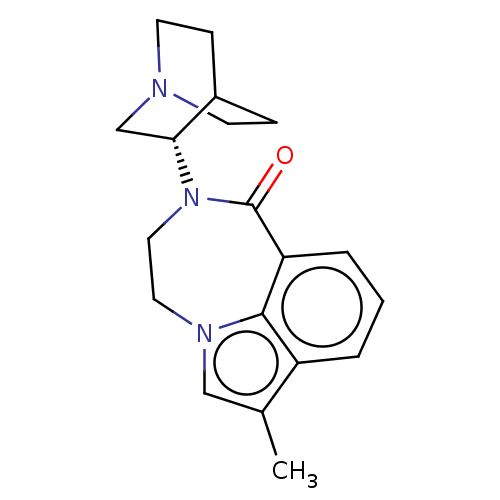 (US9045501, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5 | -47.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinity of the various compounds for the human 5-HT3 receptor was measured in a radioligand binding assay, using a scintillation proxim... | US Patent US9045501 (2015) BindingDB Entry DOI: 10.7270/Q2MK6BN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM160714 (US9045501, 9) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 6 | -46.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinity of the various compounds for the human 5-HT3 receptor was measured in a radioligand binding assay, using a scintillation proxim... | US Patent US9045501 (2015) BindingDB Entry DOI: 10.7270/Q2MK6BN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50014556 (CHEMBL3261486 | US9045501, 25) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 7 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinity of the various compounds for the human 5-HT3 receptor was measured in a radioligand binding assay, using a scintillation proxim... | US Patent US9045501 (2015) BindingDB Entry DOI: 10.7270/Q2MK6BN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM160730 (US9045501, 27) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 8 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinity of the various compounds for the human 5-HT3 receptor was measured in a radioligand binding assay, using a scintillation proxim... | US Patent US9045501 (2015) BindingDB Entry DOI: 10.7270/Q2MK6BN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50014550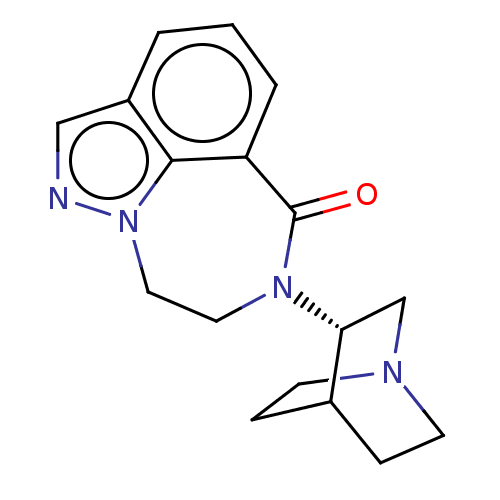 (CHEMBL3261481 | US9045501, 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 8 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinity of the various compounds for the human 5-HT3 receptor was measured in a radioligand binding assay, using a scintillation proxim... | US Patent US9045501 (2015) BindingDB Entry DOI: 10.7270/Q2MK6BN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM160715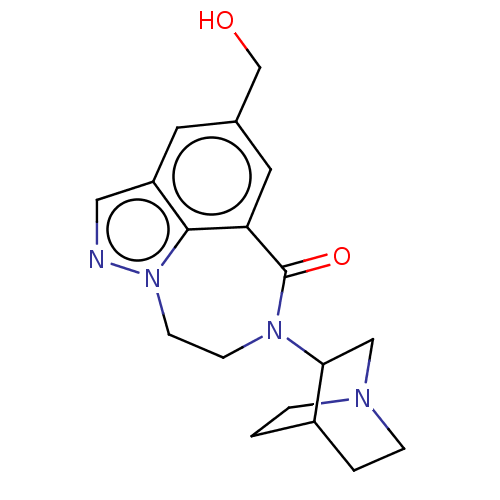 (US9045501, 10) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 14 | -44.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinity of the various compounds for the human 5-HT3 receptor was measured in a radioligand binding assay, using a scintillation proxim... | US Patent US9045501 (2015) BindingDB Entry DOI: 10.7270/Q2MK6BN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM160733 (US9045501, 30 (Enant. A) | US9045501, 31 (Enant. B...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 24 | -43.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinity of the various compounds for the human 5-HT3 receptor was measured in a radioligand binding assay, using a scintillation proxim... | US Patent US9045501 (2015) BindingDB Entry DOI: 10.7270/Q2MK6BN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM160731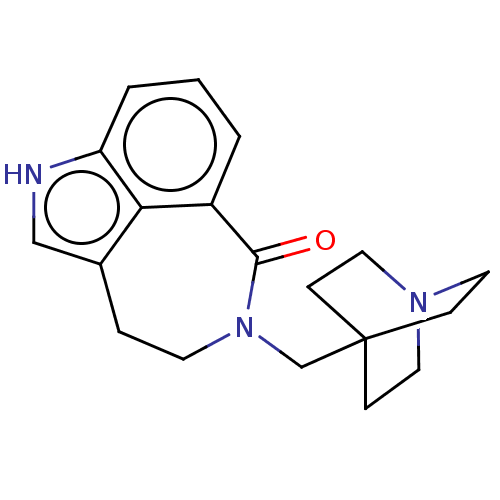 (US9045501, 28) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 30 | -42.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinity of the various compounds for the human 5-HT3 receptor was measured in a radioligand binding assay, using a scintillation proxim... | US Patent US9045501 (2015) BindingDB Entry DOI: 10.7270/Q2MK6BN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM160718 (US9045501, 13) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 32 | -42.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinity of the various compounds for the human 5-HT3 receptor was measured in a radioligand binding assay, using a scintillation proxim... | US Patent US9045501 (2015) BindingDB Entry DOI: 10.7270/Q2MK6BN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM160734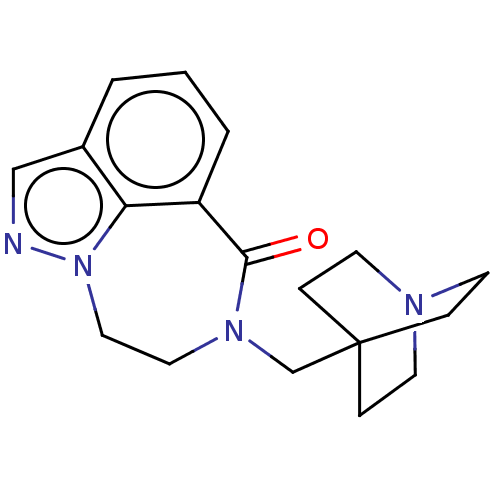 (US9045501, 32) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 73 | -40.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinity of the various compounds for the human 5-HT3 receptor was measured in a radioligand binding assay, using a scintillation proxim... | US Patent US9045501 (2015) BindingDB Entry DOI: 10.7270/Q2MK6BN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50014547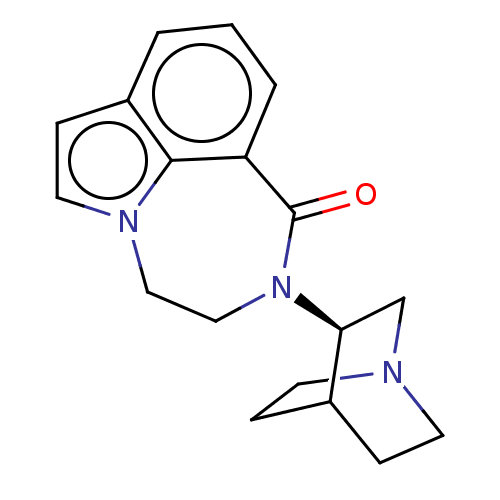 (CHEMBL3261478 | US9045501, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 74 | -40.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinity of the various compounds for the human 5-HT3 receptor was measured in a radioligand binding assay, using a scintillation proxim... | US Patent US9045501 (2015) BindingDB Entry DOI: 10.7270/Q2MK6BN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM160722 (US9045501, 17) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 130 | -39.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinity of the various compounds for the human 5-HT3 receptor was measured in a radioligand binding assay, using a scintillation proxim... | US Patent US9045501 (2015) BindingDB Entry DOI: 10.7270/Q2MK6BN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM160713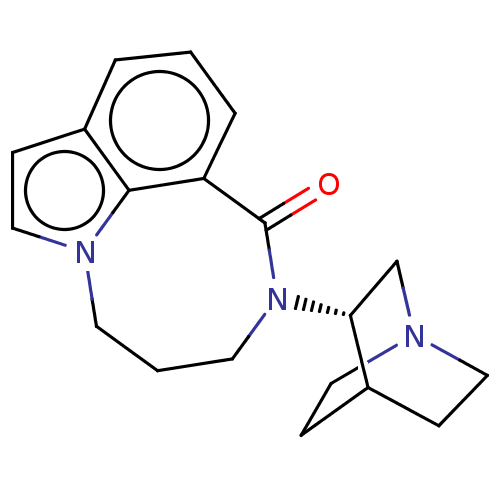 (US9045501, 6) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 205 | -38.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinity of the various compounds for the human 5-HT3 receptor was measured in a radioligand binding assay, using a scintillation proxim... | US Patent US9045501 (2015) BindingDB Entry DOI: 10.7270/Q2MK6BN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM160728 (US9045501, 23) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 206 | -38.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinity of the various compounds for the human 5-HT3 receptor was measured in a radioligand binding assay, using a scintillation proxim... | US Patent US9045501 (2015) BindingDB Entry DOI: 10.7270/Q2MK6BN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM160733 (US9045501, 30 (Enant. A) | US9045501, 31 (Enant. B...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 217 | -38.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinity of the various compounds for the human 5-HT3 receptor was measured in a radioligand binding assay, using a scintillation proxim... | US Patent US9045501 (2015) BindingDB Entry DOI: 10.7270/Q2MK6BN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM160735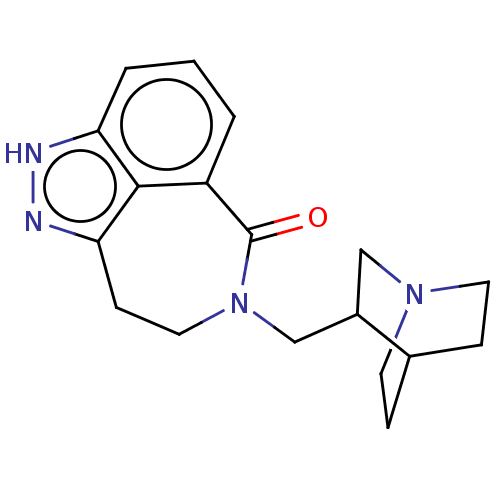 (US9045501, 33 (Enant. A) | US9045501, 34 (Enant. B...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 241 | -37.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinity of the various compounds for the human 5-HT3 receptor was measured in a radioligand binding assay, using a scintillation proxim... | US Patent US9045501 (2015) BindingDB Entry DOI: 10.7270/Q2MK6BN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM160726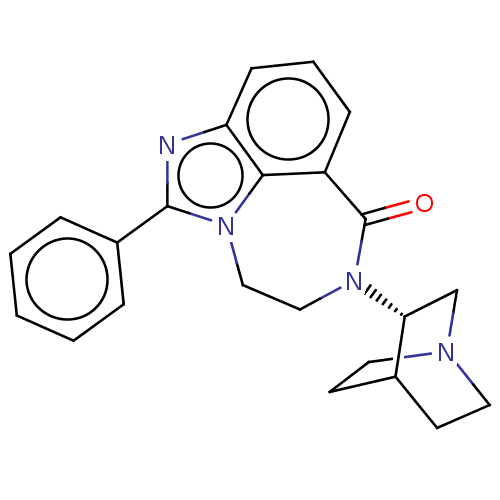 (US9045501, 21) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 406 | -36.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinity of the various compounds for the human 5-HT3 receptor was measured in a radioligand binding assay, using a scintillation proxim... | US Patent US9045501 (2015) BindingDB Entry DOI: 10.7270/Q2MK6BN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM160712 (US9045501, 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 416 | -36.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinity of the various compounds for the human 5-HT3 receptor was measured in a radioligand binding assay, using a scintillation proxim... | US Patent US9045501 (2015) BindingDB Entry DOI: 10.7270/Q2MK6BN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM160735 (US9045501, 33 (Enant. A) | US9045501, 34 (Enant. B...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 579 | -35.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinity of the various compounds for the human 5-HT3 receptor was measured in a radioligand binding assay, using a scintillation proxim... | US Patent US9045501 (2015) BindingDB Entry DOI: 10.7270/Q2MK6BN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50014557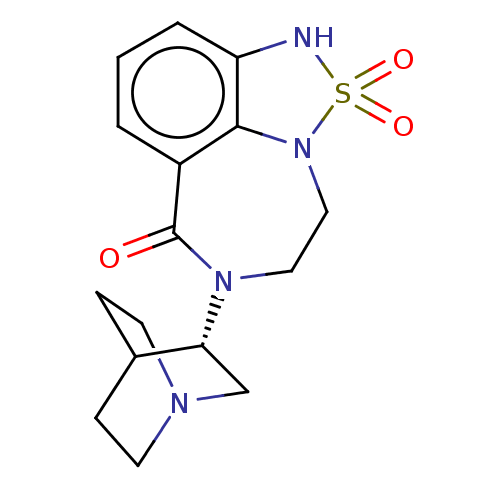 (CHEMBL3261487 | US9045501, 26) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 701 | -35.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinity of the various compounds for the human 5-HT3 receptor was measured in a radioligand binding assay, using a scintillation proxim... | US Patent US9045501 (2015) BindingDB Entry DOI: 10.7270/Q2MK6BN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||