

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166333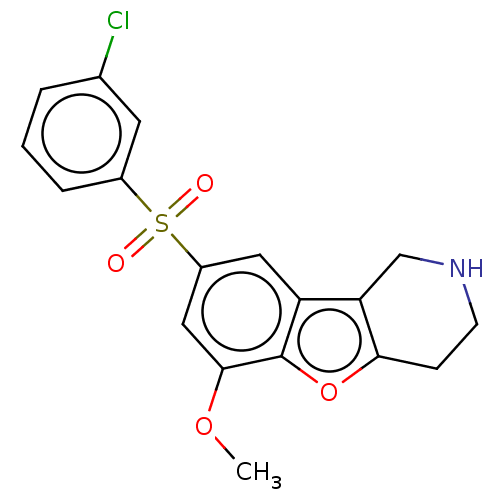 (US9067949, 190) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | -58.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166213 (US9067949, 70) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0830 | -57.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166328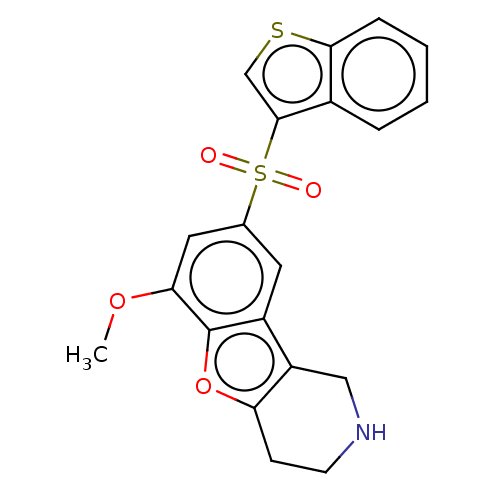 (US9067949, 185) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | -57.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166358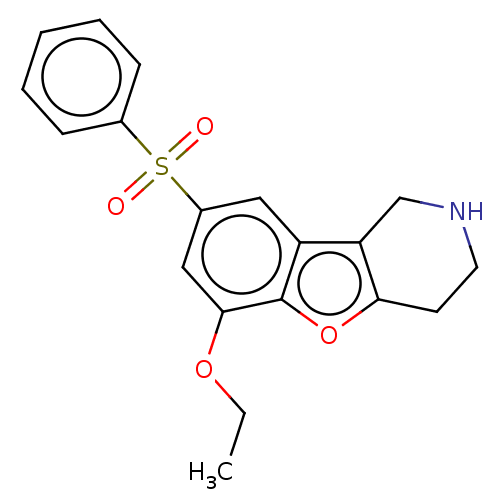 (US9067949, 215) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.110 | -56.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166331 (US9067949, 188) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.110 | -56.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166303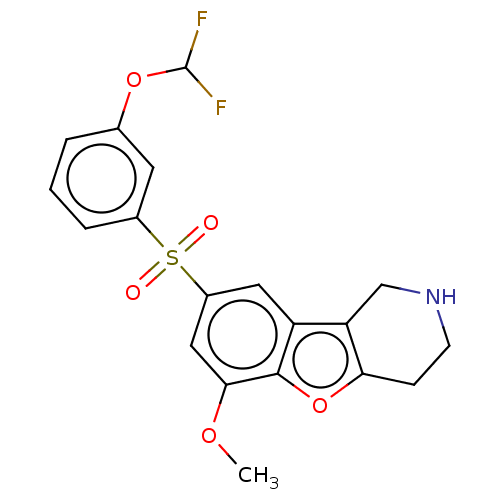 (US9067949, 160) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.110 | -56.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166197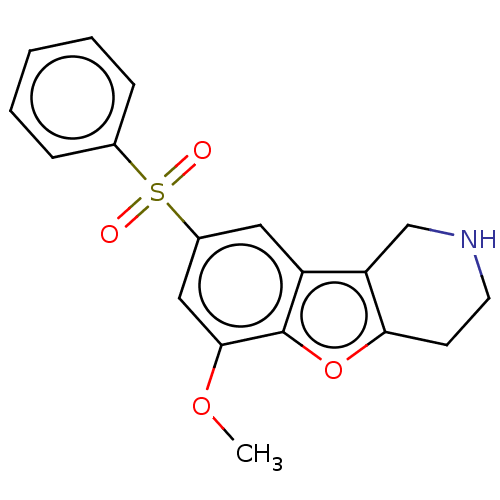 (US9067949, 54) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.120 | -56.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166244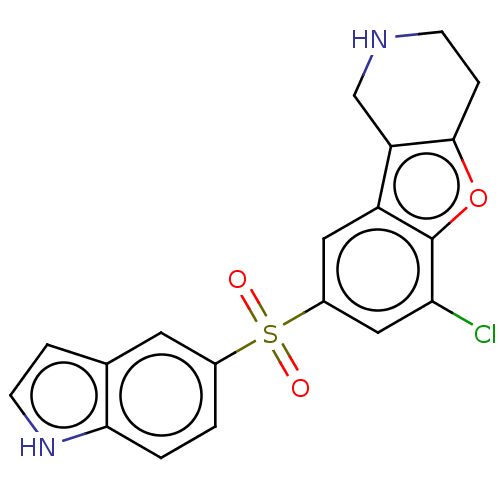 (US9067949, 101 | US9067949, 143) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.130 | -56.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166244 (US9067949, 101 | US9067949, 143) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.130 | -56.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166310 (US9067949, 167) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.130 | -56.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166225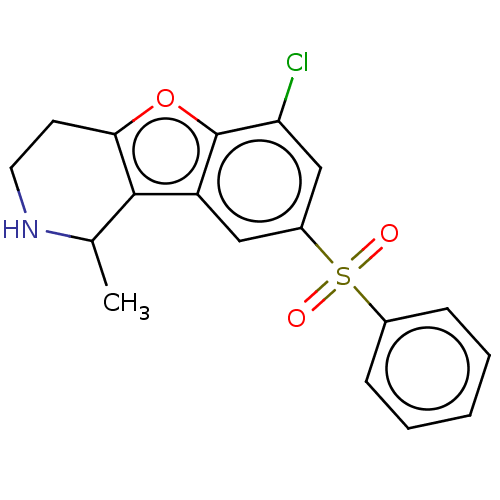 (US9067949, 81a | US9067949, 82a | US9067949, 82b) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.150 | -56.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166327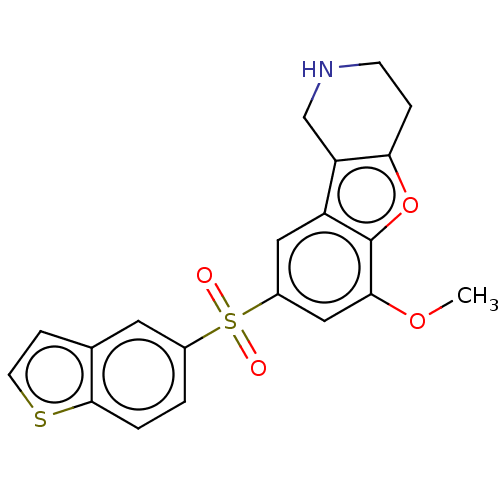 (US9067949, 184) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.160 | -55.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166326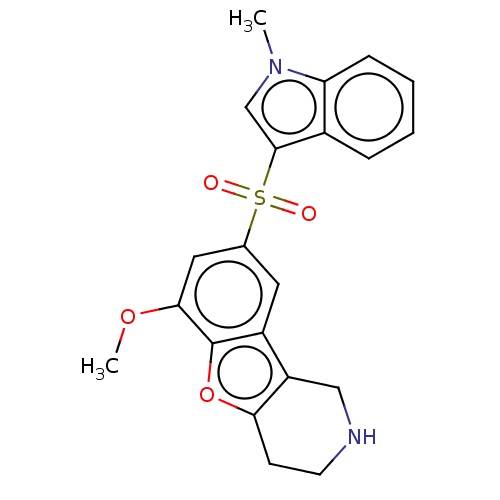 (US9067949, 183) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.160 | -55.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166193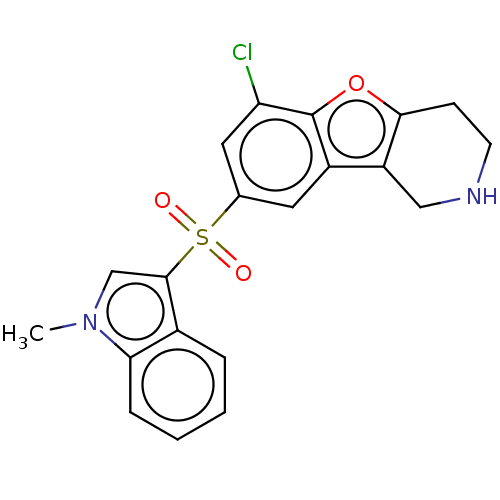 (US9067949, 50) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.170 | -55.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166335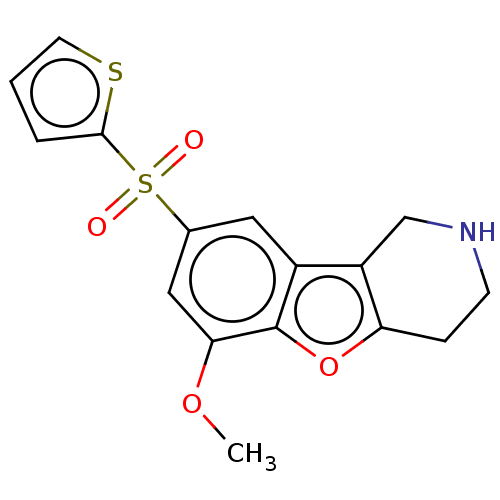 (US9067949, 192) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.170 | -55.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166311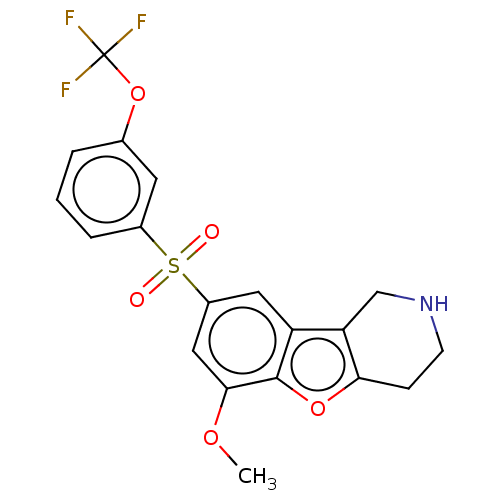 (US9067949, 168) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.190 | -55.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166306 (US9067949, 163) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.190 | -55.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166309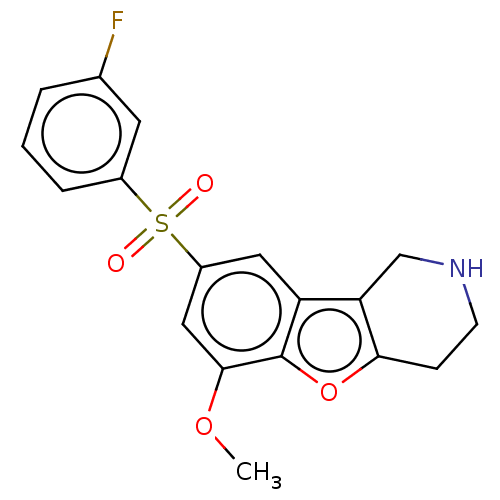 (US9067949, 166) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.200 | -55.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166194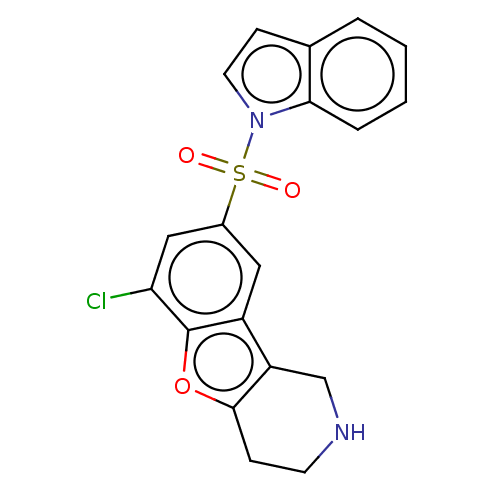 (US9067949, 51) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.200 | -55.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166344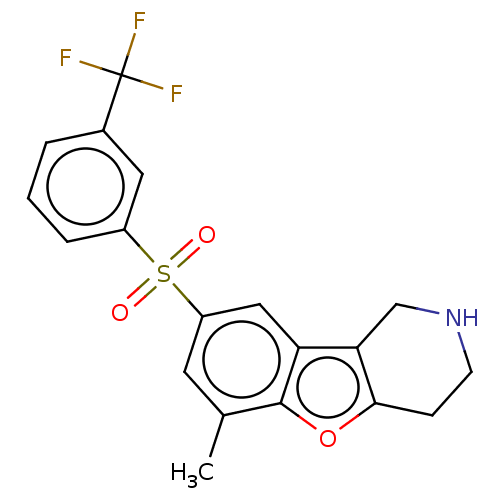 (US9067949, 201) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.220 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166196 (US9067949, 53) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.220 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166347 (US9067949, 204) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.230 | -55.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166334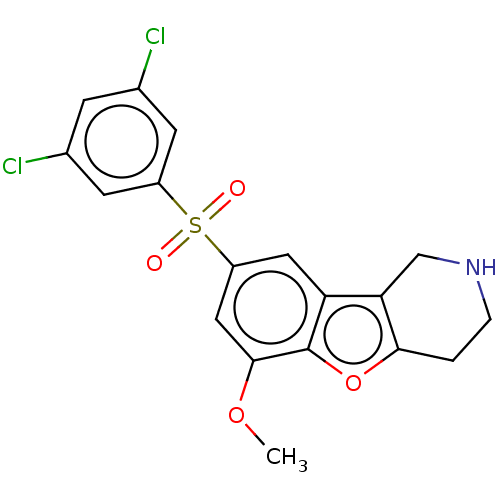 (US9067949, 191) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.230 | -55.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166233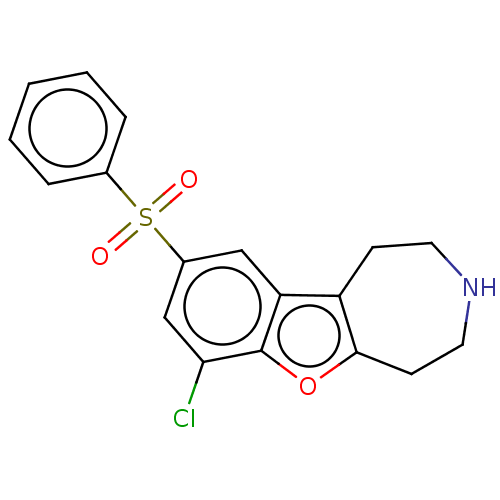 (US9067949, 90) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.25 | -54.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166276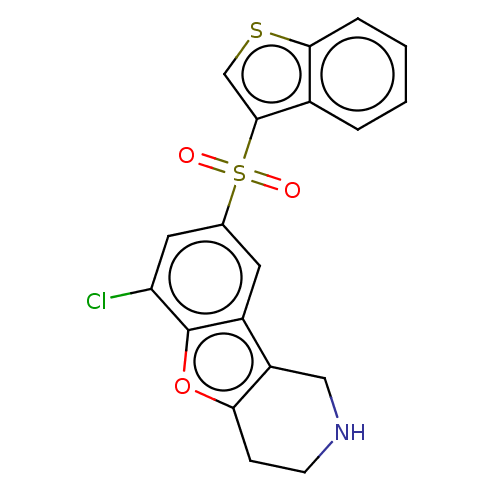 (US9067949, 133) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.260 | -54.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166330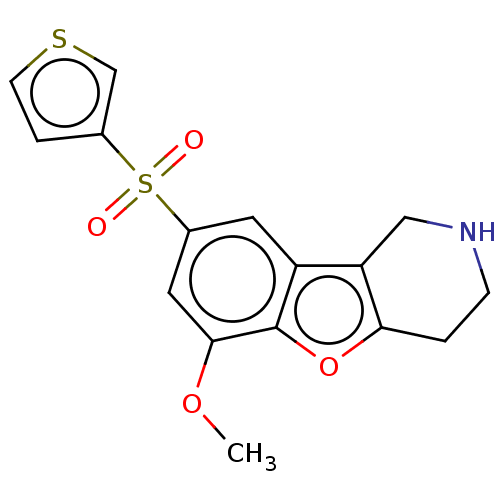 (US9067949, 187) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.270 | -54.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166338 (US9067949, 195) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.280 | -54.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166332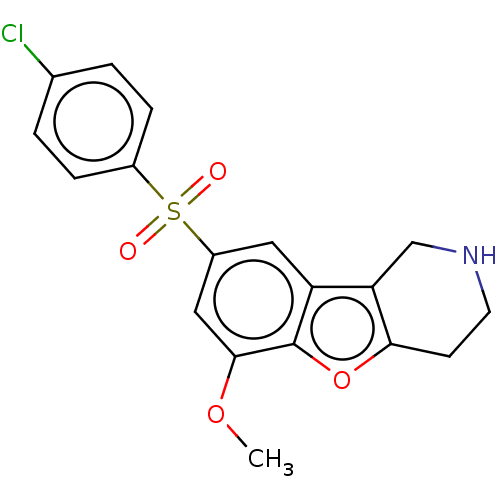 (US9067949, 189) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.280 | -54.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166345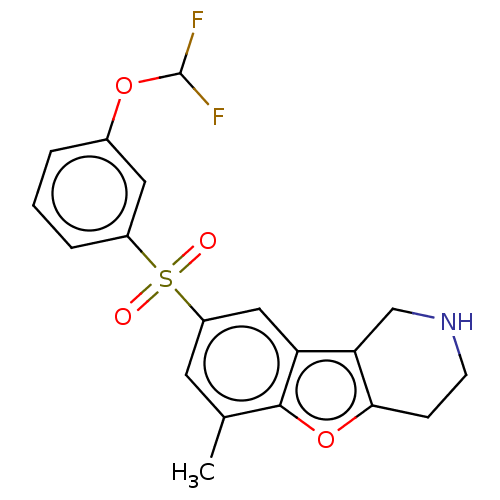 (US9067949, 202) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | -54.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166321 (US9067949, 178) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.320 | -54.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166304 (US9067949, 161) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.320 | -54.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166359 (US9067949, 216) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.330 | -54.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166329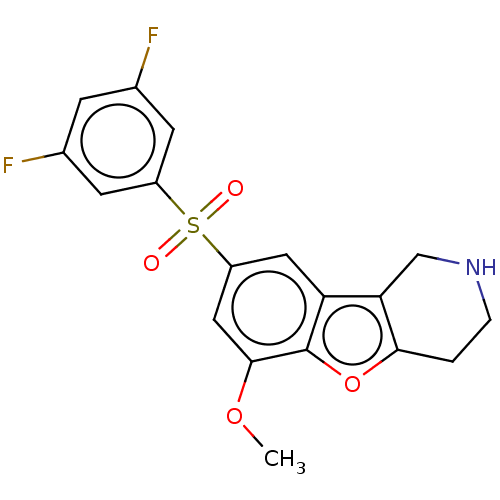 (US9067949, 186) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.330 | -54.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166320 (US9067949, 177) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.350 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166189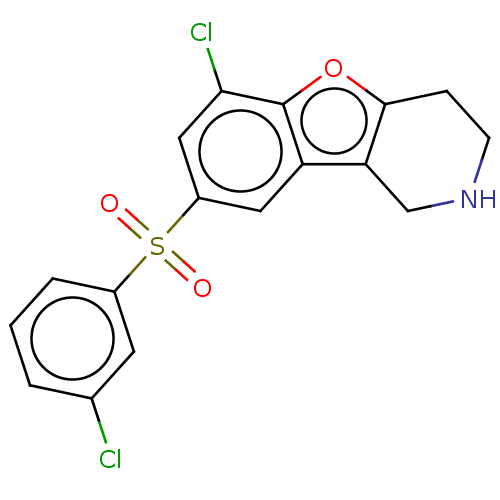 (US9067949, 46) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.350 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166225 (US9067949, 81a | US9067949, 82a | US9067949, 82b) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.350 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166322 (US9067949, 179 | US9067949, 181 | US9067949, 182) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.360 | -53.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166190 (US9067949, 47) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.390 | -53.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166337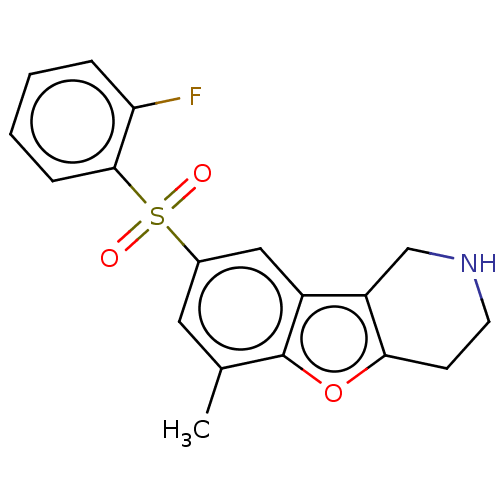 (US9067949, 194) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.390 | -53.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166354 (US9067949, 211) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166307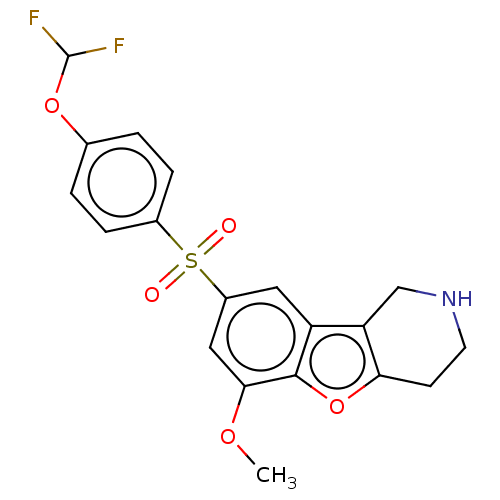 (US9067949, 164) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166322 (US9067949, 179 | US9067949, 181 | US9067949, 182) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.410 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166186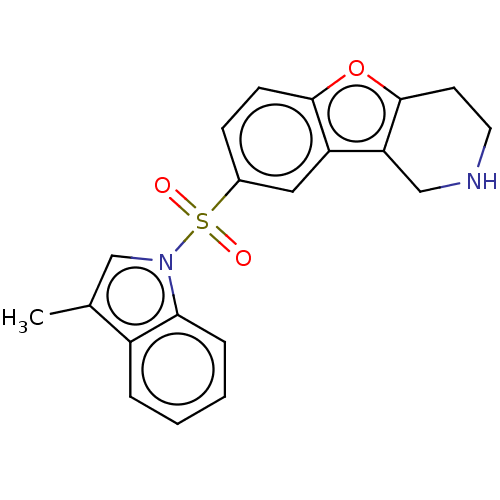 (US9067949, 43) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.420 | -53.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166188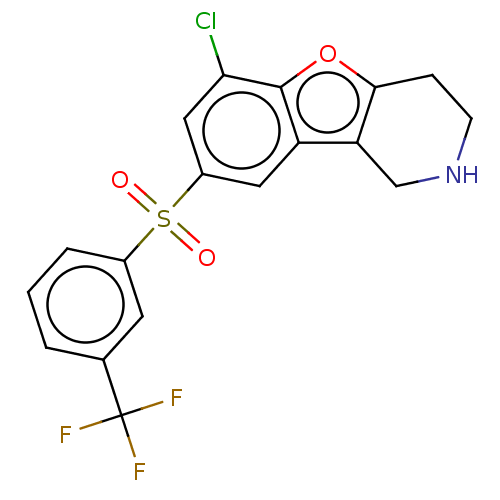 (US9067949, 45) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.480 | -53.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166301 (US9067949, 158) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.480 | -53.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166305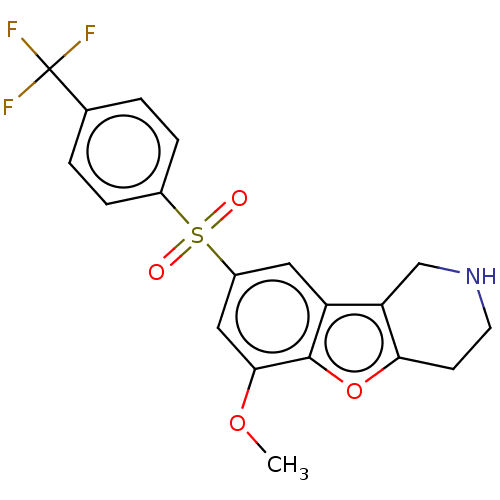 (US9067949, 162) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.490 | -53.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166322 (US9067949, 179 | US9067949, 181 | US9067949, 182) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.490 | -53.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166343 (US9067949, 200) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166187 (US9067949, 44) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.550 | -52.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM166277 (US9067949, 134) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.550 | -52.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Albany Molecular Research, Inc. US Patent | Assay Description For binding analysis vs. the human receptor, samples were incubated in 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 1 mM EDTA (4% DMSO final) with 10 nM [N-me... | US Patent US9067949 (2015) BindingDB Entry DOI: 10.7270/Q23777FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 216 total ) | Next | Last >> |