

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM168618 (US9079852, Table F, Compound 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 553 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM168620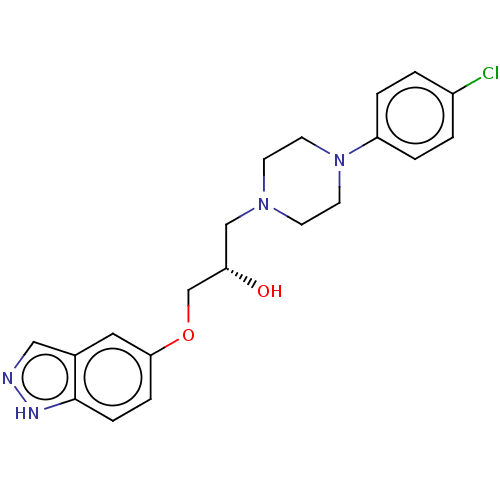 (US9079852, Table F, Compound 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM168617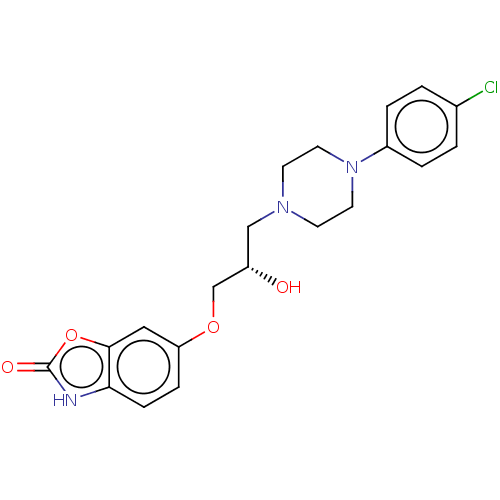 (US9079852, Table F, Compound 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM168626 (US9079852, Table F, Compound 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM168625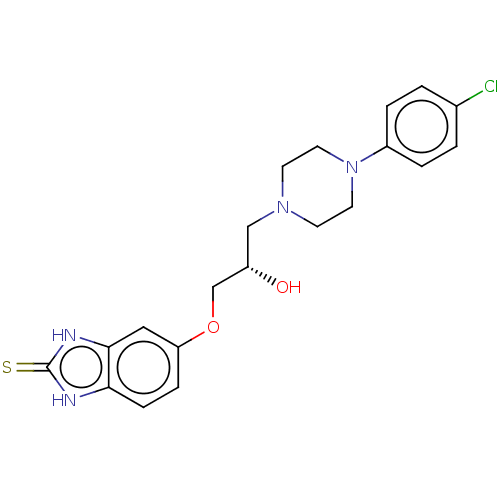 (US9079852, Table F, Compound 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM168621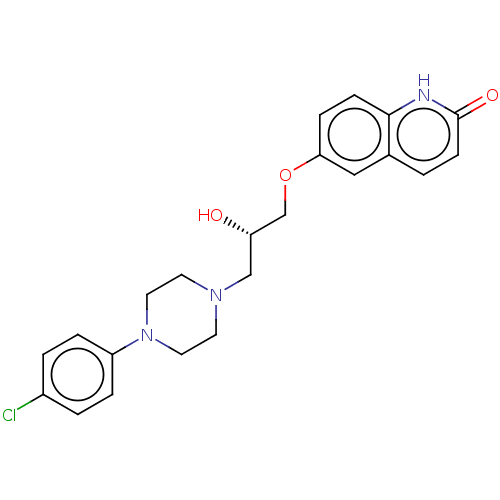 (US9079852, Table F, Compound 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM168622 (US9079852, Table F, Compound 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM168624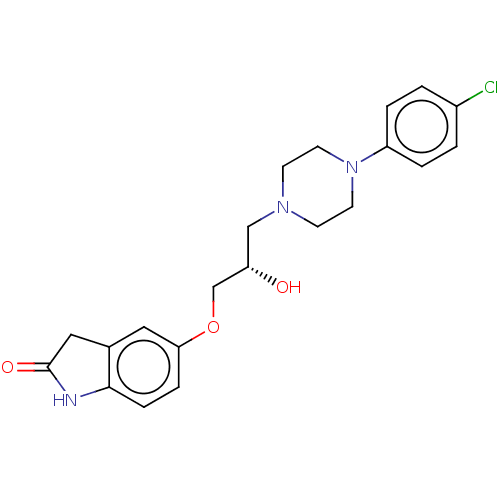 (US9079852, Table F, Compound 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM168619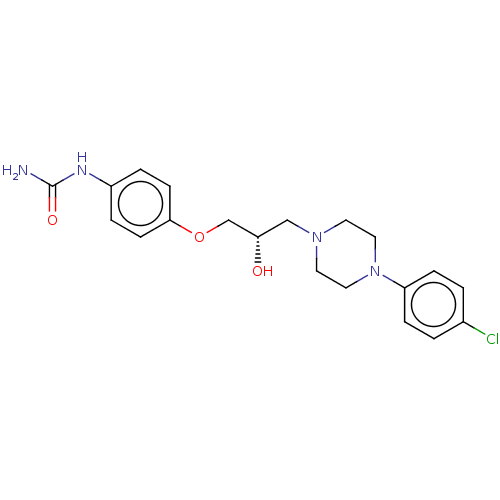 (US9079852, Table F, Compound 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM168616 (US9079852, Table F, Compound 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Compounds were evaluated for binding to the human ether-a-go-go potassium channel (hERG) expressed in HEK293 cells by displacement of 3[H]-astemizole... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM168624 (US9079852, Table F, Compound 9) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Binding to the rat alpha-1 adrenergic receptor in rat brain membranes was determined by displacement of 3[H]-prazosin (P. Greengrass and R. Bremner; ... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM168626 (US9079852, Table F, Compound 11) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Binding to the rat alpha-1 adrenergic receptor in rat brain membranes was determined by displacement of 3[H]-prazosin (P. Greengrass and R. Bremner; ... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM168625 (US9079852, Table F, Compound 10) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Binding to the rat alpha-1 adrenergic receptor in rat brain membranes was determined by displacement of 3[H]-prazosin (P. Greengrass and R. Bremner; ... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM168620 (US9079852, Table F, Compound 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Binding to the rat alpha-1 adrenergic receptor in rat brain membranes was determined by displacement of 3[H]-prazosin (P. Greengrass and R. Bremner; ... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM168619 (US9079852, Table F, Compound 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Binding to the rat alpha-1 adrenergic receptor in rat brain membranes was determined by displacement of 3[H]-prazosin (P. Greengrass and R. Bremner; ... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM168618 (US9079852, Table F, Compound 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Binding to the rat alpha-1 adrenergic receptor in rat brain membranes was determined by displacement of 3[H]-prazosin (P. Greengrass and R. Bremner; ... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM168621 (US9079852, Table F, Compound 6) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Binding to the rat alpha-1 adrenergic receptor in rat brain membranes was determined by displacement of 3[H]-prazosin (P. Greengrass and R. Bremner; ... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM168622 (US9079852, Table F, Compound 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Binding to the rat alpha-1 adrenergic receptor in rat brain membranes was determined by displacement of 3[H]-prazosin (P. Greengrass and R. Bremner; ... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM168617 (US9079852, Table F, Compound 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Binding to the rat alpha-1 adrenergic receptor in rat brain membranes was determined by displacement of 3[H]-prazosin (P. Greengrass and R. Bremner; ... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM168616 (US9079852, Table F, Compound 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University; NeurOp, Inc. US Patent | Assay Description Binding to the rat alpha-1 adrenergic receptor in rat brain membranes was determined by displacement of 3[H]-prazosin (P. Greengrass and R. Bremner; ... | US Patent US9079852 (2015) BindingDB Entry DOI: 10.7270/Q2K35SF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||