

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM173484 (US9096606, 21) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.190 | -51.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purdue Pharma, L.P. US Patent | Assay Description Membranes from recombinant HEK-293 cells expressing the human kippa opioid receptor (cloned in house) were prepared by lysing cells in ice cold hypot... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM173479 (US9096606, 16) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.200 | -51.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purdue Pharma, L.P. US Patent | Assay Description Membranes from recombinant HEK-293 cells expressing the human kippa opioid receptor (cloned in house) were prepared by lysing cells in ice cold hypot... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM173478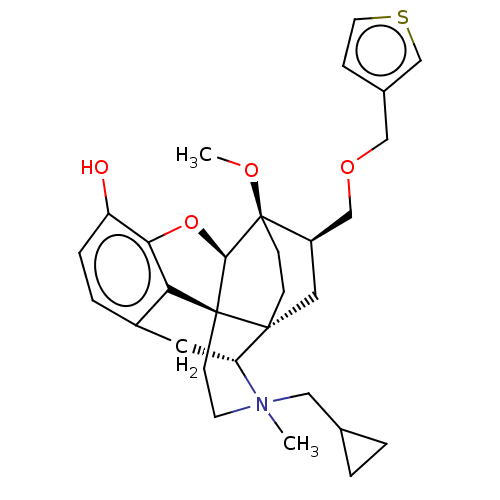 (US9096606, 15) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.200 | -51.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purdue Pharma, L.P. US Patent | Assay Description Membranes from recombinant HEK-293 cells expressing the human kippa opioid receptor (cloned in house) were prepared by lysing cells in ice cold hypot... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM173482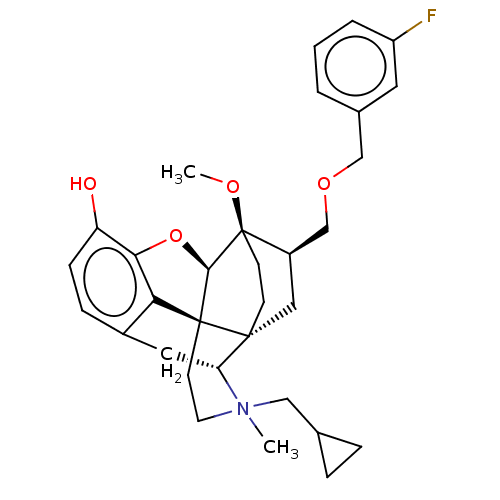 (US9096606, 19) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.25 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purdue Pharma, L.P. US Patent | Assay Description Membranes from recombinant HEK-293 cells expressing the human kippa opioid receptor (cloned in house) were prepared by lysing cells in ice cold hypot... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM173467 (US9096606, 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.260 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purdue Pharma, L.P. US Patent | Assay Description Membranes from recombinant HEK-293 cells expressing the human kippa opioid receptor (cloned in house) were prepared by lysing cells in ice cold hypot... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM173483 (US9096606, 20) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.270 | -50.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purdue Pharma, L.P. US Patent | Assay Description Membranes from recombinant HEK-293 cells expressing the human kippa opioid receptor (cloned in house) were prepared by lysing cells in ice cold hypot... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM173474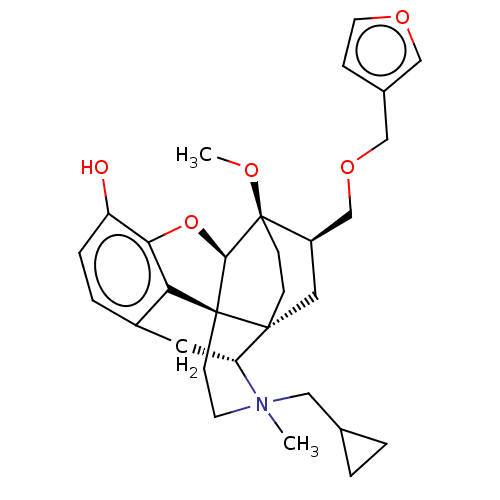 (US9096606, 11) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.290 | -50.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purdue Pharma, L.P. US Patent | Assay Description Membranes from recombinant HEK-293 cells expressing the human kippa opioid receptor (cloned in house) were prepared by lysing cells in ice cold hypot... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM173480 (US9096606, 17) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.290 | -50.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purdue Pharma, L.P. US Patent | Assay Description Membranes from recombinant HEK-293 cells expressing the human kippa opioid receptor (cloned in house) were prepared by lysing cells in ice cold hypot... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM173485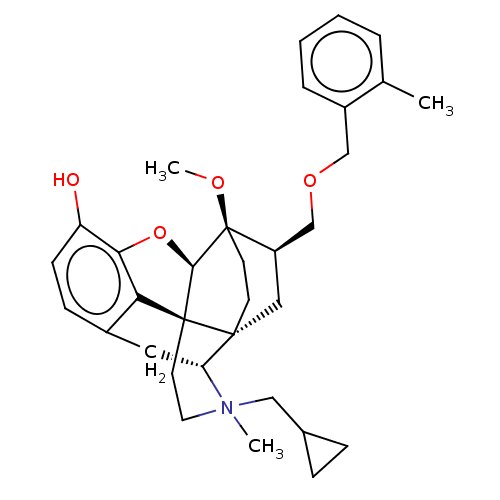 (US9096606, 22) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.470 | -49.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purdue Pharma, L.P. US Patent | Assay Description Membranes from recombinant HEK-293 cells expressing the human kippa opioid receptor (cloned in house) were prepared by lysing cells in ice cold hypot... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM173470 (US9096606, 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.550 | -49.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purdue Pharma, L.P. US Patent | Assay Description Membranes from recombinant HEK-293 cells expressing the human kippa opioid receptor (cloned in house) were prepared by lysing cells in ice cold hypot... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM173477 (US9096606, 14) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.590 | -49.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purdue Pharma, L.P. US Patent | Assay Description Membranes from recombinant HEK-293 cells expressing the human kippa opioid receptor (cloned in house) were prepared by lysing cells in ice cold hypot... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM173468 (US9096606, 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.780 | -48.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purdue Pharma, L.P. US Patent | Assay Description Membranes from recombinant HEK-293 cells expressing the human kippa opioid receptor (cloned in house) were prepared by lysing cells in ice cold hypot... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM173469 (US9096606, 6) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.06 | -47.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purdue Pharma, L.P. US Patent | Assay Description Membranes from recombinant HEK-293 cells expressing the human kippa opioid receptor (cloned in house) were prepared by lysing cells in ice cold hypot... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM173469 (US9096606, 6) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.36 | -54.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Purdue Pharma, L.P. US Patent | Assay Description Radioligand dose-displacement binding assays for -opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membrane ... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM173470 (US9096606, 7) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.11 | -53.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Purdue Pharma, L.P. US Patent | Assay Description Radioligand dose-displacement binding assays for -opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membrane ... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM173468 (US9096606, 5) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.29 | -53.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Purdue Pharma, L.P. US Patent | Assay Description Radioligand dose-displacement binding assays for -opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membrane ... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM173480 (US9096606, 17) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.30 | -51.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Purdue Pharma, L.P. US Patent | Assay Description Radioligand dose-displacement binding assays for -opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membrane ... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM173479 (US9096606, 16) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.51 | -51.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Purdue Pharma, L.P. US Patent | Assay Description Radioligand dose-displacement binding assays for -opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membrane ... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM173464 (US9096606, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5.02 | -44.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purdue Pharma, L.P. US Patent | Assay Description Membranes from recombinant HEK-293 cells expressing the human kippa opioid receptor (cloned in house) were prepared by lysing cells in ice cold hypot... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM173482 (US9096606, 19) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5.35 | -51.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Purdue Pharma, L.P. US Patent | Assay Description Radioligand dose-displacement binding assays for -opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membrane ... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM173483 (US9096606, 20) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 6.26 | -50.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Purdue Pharma, L.P. US Patent | Assay Description Radioligand dose-displacement binding assays for -opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membrane ... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM173467 (US9096606, 4) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 9.55 | -49.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Purdue Pharma, L.P. US Patent | Assay Description Radioligand dose-displacement binding assays for -opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membrane ... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM173487 (US9096606, 24) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 10.6 | -42.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purdue Pharma, L.P. US Patent | Assay Description Membranes from recombinant HEK-293 cells expressing the human kippa opioid receptor (cloned in house) were prepared by lysing cells in ice cold hypot... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM173475 (US9096606, 12) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 17.3 | -41.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purdue Pharma, L.P. US Patent | Assay Description Membranes from recombinant HEK-293 cells expressing the human kippa opioid receptor (cloned in house) were prepared by lysing cells in ice cold hypot... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM173470 (US9096606, 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 18.8 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma, L.P. US Patent | Assay Description δ-opioid Receptor Binding Assay Procedures were conducted as follows. Radioligand dose-displacement assays used 0.3 nM [3H]-Naltrindole (Perkin ... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM173478 (US9096606, 15) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 24.3 | -47.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Purdue Pharma, L.P. US Patent | Assay Description Radioligand dose-displacement binding assays for -opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membrane ... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM173473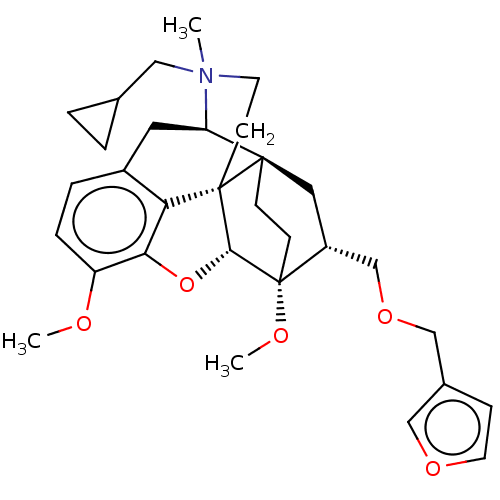 (US9096606, 10) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 35.0 | -39.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purdue Pharma, L.P. US Patent | Assay Description Membranes from recombinant HEK-293 cells expressing the human kippa opioid receptor (cloned in house) were prepared by lysing cells in ice cold hypot... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM173468 (US9096606, 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 45.3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma, L.P. US Patent | Assay Description δ-opioid Receptor Binding Assay Procedures were conducted as follows. Radioligand dose-displacement assays used 0.3 nM [3H]-Naltrindole (Perkin ... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM173474 (US9096606, 11) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 47.1 | -45.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Purdue Pharma, L.P. US Patent | Assay Description Radioligand dose-displacement binding assays for -opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membrane ... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM173471 (US9096606, 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 57.0 | -38.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purdue Pharma, L.P. US Patent | Assay Description Membranes from recombinant HEK-293 cells expressing the human kippa opioid receptor (cloned in house) were prepared by lysing cells in ice cold hypot... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM173465 (US9096606, 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 66.7 | -38.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purdue Pharma, L.P. US Patent | Assay Description Membranes from recombinant HEK-293 cells expressing the human kippa opioid receptor (cloned in house) were prepared by lysing cells in ice cold hypot... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM173464 (US9096606, 1) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 67.2 | -44.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Purdue Pharma, L.P. US Patent | Assay Description Radioligand dose-displacement binding assays for -opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membrane ... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM173469 (US9096606, 6) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 109 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma, L.P. US Patent | Assay Description δ-opioid Receptor Binding Assay Procedures were conducted as follows. Radioligand dose-displacement assays used 0.3 nM [3H]-Naltrindole (Perkin ... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM173486 (US9096606, 23) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 112 | -36.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purdue Pharma, L.P. US Patent | Assay Description Membranes from recombinant HEK-293 cells expressing the human kippa opioid receptor (cloned in house) were prepared by lysing cells in ice cold hypot... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM173466 (US9096606, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 117 | -36.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purdue Pharma, L.P. US Patent | Assay Description Membranes from recombinant HEK-293 cells expressing the human kippa opioid receptor (cloned in house) were prepared by lysing cells in ice cold hypot... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM173465 (US9096606, 2) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 163 | -42.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Purdue Pharma, L.P. US Patent | Assay Description Radioligand dose-displacement binding assays for -opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membrane ... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM173476 (US9096606, 13) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 165 | -36.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purdue Pharma, L.P. US Patent | Assay Description Membranes from recombinant HEK-293 cells expressing the human kippa opioid receptor (cloned in house) were prepared by lysing cells in ice cold hypot... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM173467 (US9096606, 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 175 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma, L.P. US Patent | Assay Description δ-opioid Receptor Binding Assay Procedures were conducted as follows. Radioligand dose-displacement assays used 0.3 nM [3H]-Naltrindole (Perkin ... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM173476 (US9096606, 13) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 182 | -41.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Purdue Pharma, L.P. US Patent | Assay Description Radioligand dose-displacement binding assays for -opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membrane ... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM173474 (US9096606, 11) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 193 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma, L.P. US Patent | Assay Description δ-opioid Receptor Binding Assay Procedures were conducted as follows. Radioligand dose-displacement assays used 0.3 nM [3H]-Naltrindole (Perkin ... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM173478 (US9096606, 15) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 202 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma, L.P. US Patent | Assay Description δ-opioid Receptor Binding Assay Procedures were conducted as follows. Radioligand dose-displacement assays used 0.3 nM [3H]-Naltrindole (Perkin ... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM173466 (US9096606, 3) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 242 | -40.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Purdue Pharma, L.P. US Patent | Assay Description Radioligand dose-displacement binding assays for -opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membrane ... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM173486 (US9096606, 23) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 262 | -40.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Purdue Pharma, L.P. US Patent | Assay Description Radioligand dose-displacement binding assays for -opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membrane ... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM173475 (US9096606, 12) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 275 | -40.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Purdue Pharma, L.P. US Patent | Assay Description Radioligand dose-displacement binding assays for -opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membrane ... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM173472 (US9096606, 9) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 348 | -34.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purdue Pharma, L.P. US Patent | Assay Description Membranes from recombinant HEK-293 cells expressing the human kippa opioid receptor (cloned in house) were prepared by lysing cells in ice cold hypot... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM173471 (US9096606, 8) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 544 | -38.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Purdue Pharma, L.P. US Patent | Assay Description Radioligand dose-displacement binding assays for -opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membrane ... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM173487 (US9096606, 24) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 571 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Purdue Pharma, L.P. US Patent | Assay Description Radioligand dose-displacement binding assays for -opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membrane ... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM173481 (US9096606, 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.03E+3 | -31.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purdue Pharma, L.P. US Patent | Assay Description Membranes from recombinant HEK-293 cells expressing the human kippa opioid receptor (cloned in house) were prepared by lysing cells in ice cold hypot... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM173472 (US9096606, 9) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.49E+3 | -36.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Purdue Pharma, L.P. US Patent | Assay Description Radioligand dose-displacement binding assays for -opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membrane ... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM173464 (US9096606, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma, L.P. US Patent | Assay Description δ-opioid Receptor Binding Assay Procedures were conducted as follows. Radioligand dose-displacement assays used 0.3 nM [3H]-Naltrindole (Perkin ... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM173487 (US9096606, 24) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma, L.P. US Patent | Assay Description δ-opioid Receptor Binding Assay Procedures were conducted as follows. Radioligand dose-displacement assays used 0.3 nM [3H]-Naltrindole (Perkin ... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM173473 (US9096606, 10) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5.81E+3 | -32.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Purdue Pharma, L.P. US Patent | Assay Description Radioligand dose-displacement binding assays for -opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membrane ... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM173466 (US9096606, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma, L.P. US Patent | Assay Description δ-opioid Receptor Binding Assay Procedures were conducted as follows. Radioligand dose-displacement assays used 0.3 nM [3H]-Naltrindole (Perkin ... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM173468 (US9096606, 5) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.82E+4 | -25.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purdue Pharma, L.P. US Patent | Assay Description Membranes from recombinant HEK-293 cells expressing the human opioid receptor-like receptor (ORL-1) (Perkin Elmer, Shelton, Conn.) were prepared by l... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM173465 (US9096606, 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma, L.P. US Patent | Assay Description δ-opioid Receptor Binding Assay Procedures were conducted as follows. Radioligand dose-displacement assays used 0.3 nM [3H]-Naltrindole (Perkin ... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM173469 (US9096606, 6) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >2.00E+4 | >-24.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purdue Pharma, L.P. US Patent | Assay Description Membranes from recombinant HEK-293 cells expressing the human opioid receptor-like receptor (ORL-1) (Perkin Elmer, Shelton, Conn.) were prepared by l... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM173466 (US9096606, 3) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >2.00E+4 | >-24.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purdue Pharma, L.P. US Patent | Assay Description Membranes from recombinant HEK-293 cells expressing the human opioid receptor-like receptor (ORL-1) (Perkin Elmer, Shelton, Conn.) were prepared by l... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM173471 (US9096606, 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma, L.P. US Patent | Assay Description δ-opioid Receptor Binding Assay Procedures were conducted as follows. Radioligand dose-displacement assays used 0.3 nM [3H]-Naltrindole (Perkin ... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM173486 (US9096606, 23) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >2.00E+4 | >-24.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purdue Pharma, L.P. US Patent | Assay Description Membranes from recombinant HEK-293 cells expressing the human opioid receptor-like receptor (ORL-1) (Perkin Elmer, Shelton, Conn.) were prepared by l... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM173465 (US9096606, 2) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >2.00E+4 | >-24.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purdue Pharma, L.P. US Patent | Assay Description Membranes from recombinant HEK-293 cells expressing the human opioid receptor-like receptor (ORL-1) (Perkin Elmer, Shelton, Conn.) were prepared by l... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM173464 (US9096606, 1) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >2.00E+4 | >-24.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purdue Pharma, L.P. US Patent | Assay Description Membranes from recombinant HEK-293 cells expressing the human opioid receptor-like receptor (ORL-1) (Perkin Elmer, Shelton, Conn.) were prepared by l... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM173467 (US9096606, 4) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >2.00E+4 | >-24.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purdue Pharma, L.P. US Patent | Assay Description Membranes from recombinant HEK-293 cells expressing the human opioid receptor-like receptor (ORL-1) (Perkin Elmer, Shelton, Conn.) were prepared by l... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM173486 (US9096606, 23) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Purdue Pharma, L.P. US Patent | Assay Description δ-opioid Receptor Binding Assay Procedures were conducted as follows. Radioligand dose-displacement assays used 0.3 nM [3H]-Naltrindole (Perkin ... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid growth factor receptor-like protein 1 (Homo sapiens (Human)) | BDBM173471 (US9096606, 8) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | >2.00E+4 | >-24.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Purdue Pharma, L.P. US Patent | Assay Description Membranes from recombinant HEK-293 cells expressing the human opioid receptor-like receptor (ORL-1) (Perkin Elmer, Shelton, Conn.) were prepared by l... | US Patent US9096606 (2015) BindingDB Entry DOI: 10.7270/Q2J101XF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||