

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM173867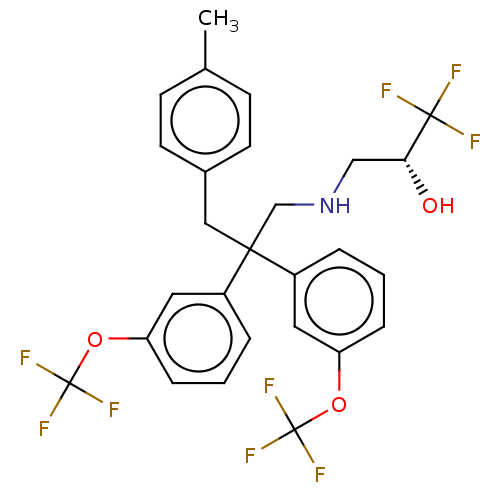 (US9102599, 50) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company US Patent | Assay Description Compounds of the present invention inhibit CETP-dependent cholesterol ester transfer from HDL to LDL as described here. Dilutions of compounds in DMS... | US Patent US9102599 (2015) BindingDB Entry DOI: 10.7270/Q2V986TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM173858 (US9102599, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company US Patent | Assay Description Compounds of the present invention inhibit CETP-dependent cholesterol ester transfer from HDL to LDL as described here. Dilutions of compounds in DMS... | US Patent US9102599 (2015) BindingDB Entry DOI: 10.7270/Q2V986TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM173866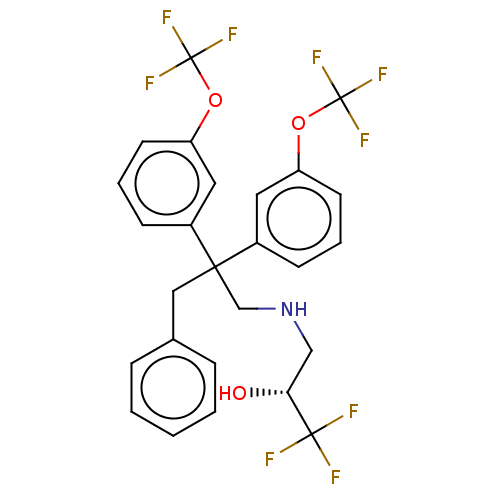 (US9102599, 47) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company US Patent | Assay Description Compounds of the present invention inhibit CETP-dependent cholesterol ester transfer from HDL to LDL as described here. Dilutions of compounds in DMS... | US Patent US9102599 (2015) BindingDB Entry DOI: 10.7270/Q2V986TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM173864 (US9102599, 44) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company US Patent | Assay Description Compounds of the present invention inhibit CETP-dependent cholesterol ester transfer from HDL to LDL as described here. Dilutions of compounds in DMS... | US Patent US9102599 (2015) BindingDB Entry DOI: 10.7270/Q2V986TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM173868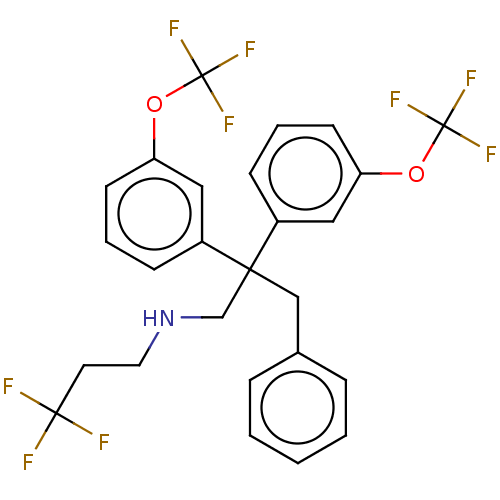 (US9102599, 53) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 25 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company US Patent | Assay Description Compounds of the present invention inhibit CETP-dependent cholesterol ester transfer from HDL to LDL as described here. Dilutions of compounds in DMS... | US Patent US9102599 (2015) BindingDB Entry DOI: 10.7270/Q2V986TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM173860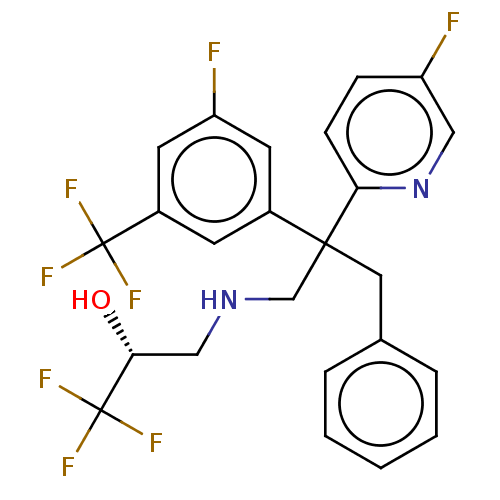 (US9102599, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company US Patent | Assay Description Compounds of the present invention inhibit CETP-dependent cholesterol ester transfer from HDL to LDL as described here. Dilutions of compounds in DMS... | US Patent US9102599 (2015) BindingDB Entry DOI: 10.7270/Q2V986TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM173871 (US9102599, 122) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 129 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company US Patent | Assay Description Compounds of the present invention inhibit CETP-dependent cholesterol ester transfer from HDL to LDL as described here. Dilutions of compounds in DMS... | US Patent US9102599 (2015) BindingDB Entry DOI: 10.7270/Q2V986TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM173862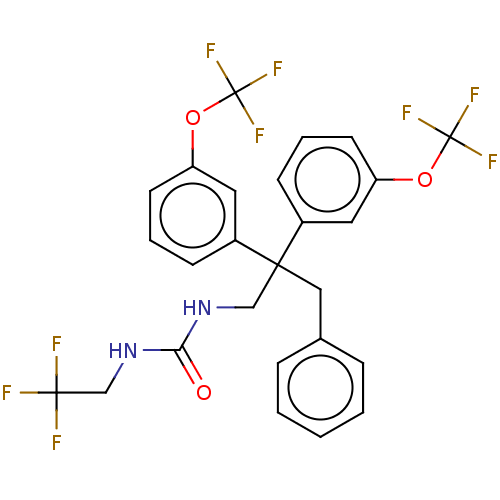 (US9102599, 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 132 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company US Patent | Assay Description Compounds of the present invention inhibit CETP-dependent cholesterol ester transfer from HDL to LDL as described here. Dilutions of compounds in DMS... | US Patent US9102599 (2015) BindingDB Entry DOI: 10.7270/Q2V986TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM173863 (US9102599, 43) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 134 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company US Patent | Assay Description Compounds of the present invention inhibit CETP-dependent cholesterol ester transfer from HDL to LDL as described here. Dilutions of compounds in DMS... | US Patent US9102599 (2015) BindingDB Entry DOI: 10.7270/Q2V986TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM173869 (US9102599, 94) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 184 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company US Patent | Assay Description Compounds of the present invention inhibit CETP-dependent cholesterol ester transfer from HDL to LDL as described here. Dilutions of compounds in DMS... | US Patent US9102599 (2015) BindingDB Entry DOI: 10.7270/Q2V986TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM173870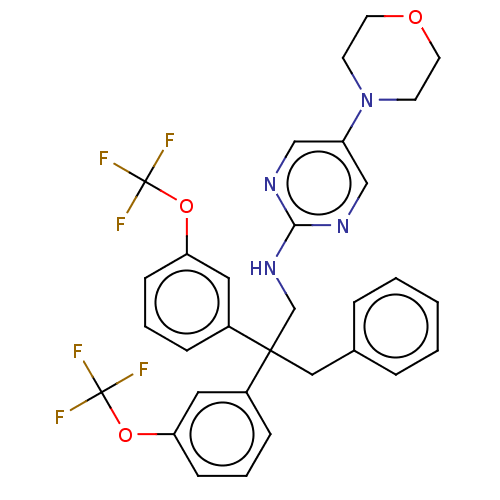 (US9102599, 112) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 248 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company US Patent | Assay Description Compounds of the present invention inhibit CETP-dependent cholesterol ester transfer from HDL to LDL as described here. Dilutions of compounds in DMS... | US Patent US9102599 (2015) BindingDB Entry DOI: 10.7270/Q2V986TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM173872 (US9102599, 133) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company US Patent | Assay Description Compounds of the present invention inhibit CETP-dependent cholesterol ester transfer from HDL to LDL as described here. Dilutions of compounds in DMS... | US Patent US9102599 (2015) BindingDB Entry DOI: 10.7270/Q2V986TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM173861 (US9102599, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.43E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company US Patent | Assay Description Compounds of the present invention inhibit CETP-dependent cholesterol ester transfer from HDL to LDL as described here. Dilutions of compounds in DMS... | US Patent US9102599 (2015) BindingDB Entry DOI: 10.7270/Q2V986TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM173865 (US9102599, 45) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.81E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company US Patent | Assay Description Compounds of the present invention inhibit CETP-dependent cholesterol ester transfer from HDL to LDL as described here. Dilutions of compounds in DMS... | US Patent US9102599 (2015) BindingDB Entry DOI: 10.7270/Q2V986TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM173859 (US9102599, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.36E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bristol-Myers Squibb Company US Patent | Assay Description Compounds of the present invention inhibit CETP-dependent cholesterol ester transfer from HDL to LDL as described here. Dilutions of compounds in DMS... | US Patent US9102599 (2015) BindingDB Entry DOI: 10.7270/Q2V986TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||