

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank US Patent | n/a | n/a | 19.4 | n/a | n/a | n/a | n/a | n/a | 25 |
Academia Sinica; National Taiwan University US Patent | Assay Description The HDAC activity was performed using the HDAC fluorescent activity assay kit (BIOMOL, Plymouth Meeting, Pa., USA) according to the manufacturer'... | US Patent US9115116 (2015) BindingDB Entry DOI: 10.7270/Q2X34W7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB US Patent | n/a | n/a | 20.9 | n/a | n/a | n/a | n/a | n/a | 25 |
Academia Sinica; National Taiwan University US Patent | Assay Description The HDAC activity was performed using the HDAC fluorescent activity assay kit (BIOMOL, Plymouth Meeting, Pa., USA) according to the manufacturer'... | US Patent US9115116 (2015) BindingDB Entry DOI: 10.7270/Q2X34W7F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM34168 (LOVASTATIN | MLS000069585 | SMR000058779 | US91151...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 29.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Academia Sinica; National Taiwan University US Patent | Assay Description The HMGR activity was performed using HMG-CoA reductase assay kit from Sigma-Aldrich with the human recombinant protein or 100 μg total cell lys... | US Patent US9115116 (2015) BindingDB Entry DOI: 10.7270/Q2X34W7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM175098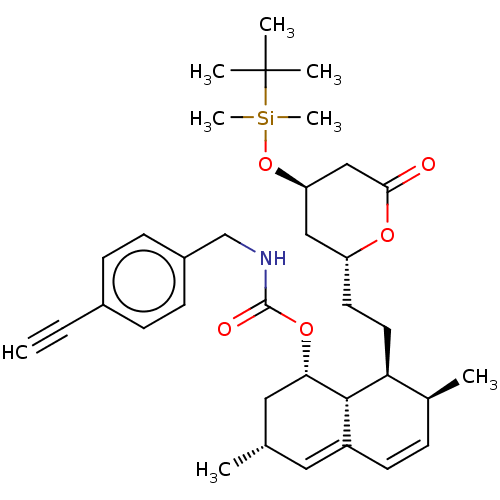 (US9115116, 6b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | 25 |
Academia Sinica; National Taiwan University US Patent | Assay Description The HDAC activity was performed using the HDAC fluorescent activity assay kit (BIOMOL, Plymouth Meeting, Pa., USA) according to the manufacturer'... | US Patent US9115116 (2015) BindingDB Entry DOI: 10.7270/Q2X34W7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50432563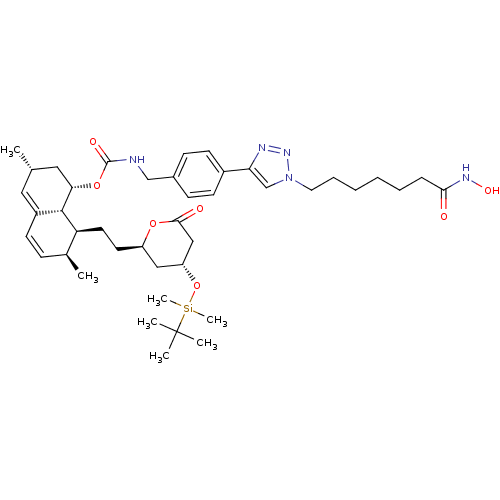 (CHEMBL2347011 | US9115116, 8b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | 25 |
Academia Sinica; National Taiwan University US Patent | Assay Description The HDAC activity was performed using the HDAC fluorescent activity assay kit (BIOMOL, Plymouth Meeting, Pa., USA) according to the manufacturer'... | US Patent US9115116 (2015) BindingDB Entry DOI: 10.7270/Q2X34W7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM175097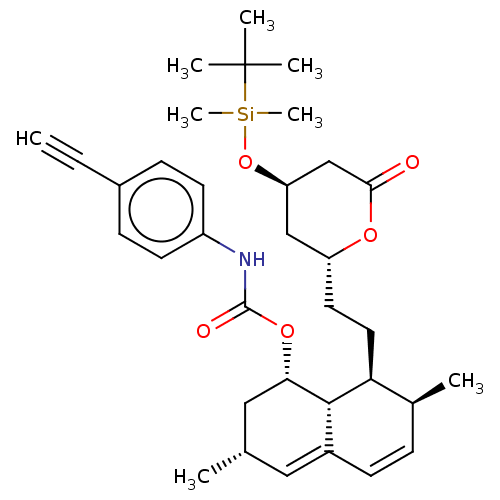 (US9115116, 6a) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 36.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Academia Sinica; National Taiwan University US Patent | Assay Description The HMGR activity was performed using HMG-CoA reductase assay kit from Sigma-Aldrich with the human recombinant protein or 100 μg total cell lys... | US Patent US9115116 (2015) BindingDB Entry DOI: 10.7270/Q2X34W7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50432564 (CHEMBL2347010 | US9115116, 8a) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 36.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Academia Sinica; National Taiwan University US Patent | Assay Description The HMGR activity was performed using HMG-CoA reductase assay kit from Sigma-Aldrich with the human recombinant protein or 100 μg total cell lys... | US Patent US9115116 (2015) BindingDB Entry DOI: 10.7270/Q2X34W7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50432563 (CHEMBL2347011 | US9115116, 8b) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 53.8 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Academia Sinica; National Taiwan University US Patent | Assay Description The HMGR activity was performed using HMG-CoA reductase assay kit from Sigma-Aldrich with the human recombinant protein or 100 μg total cell lys... | US Patent US9115116 (2015) BindingDB Entry DOI: 10.7270/Q2X34W7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM175098 (US9115116, 6b) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 53.8 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Academia Sinica; National Taiwan University US Patent | Assay Description The HMGR activity was performed using HMG-CoA reductase assay kit from Sigma-Aldrich with the human recombinant protein or 100 μg total cell lys... | US Patent US9115116 (2015) BindingDB Entry DOI: 10.7270/Q2X34W7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50432562 (CHEMBL2347005 | US9115116, 12a) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 54.1 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Academia Sinica; National Taiwan University US Patent | Assay Description The HMGR activity was performed using HMG-CoA reductase assay kit from Sigma-Aldrich with the human recombinant protein or 100 μg total cell lys... | US Patent US9115116 (2015) BindingDB Entry DOI: 10.7270/Q2X34W7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB US Patent | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | 25 |
Academia Sinica; National Taiwan University US Patent | Assay Description The HDAC activity was performed using the HDAC fluorescent activity assay kit (BIOMOL, Plymouth Meeting, Pa., USA) according to the manufacturer'... | US Patent US9115116 (2015) BindingDB Entry DOI: 10.7270/Q2X34W7F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50432562 (CHEMBL2347005 | US9115116, 12a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | 25 |
Academia Sinica; National Taiwan University US Patent | Assay Description The HDAC activity was performed using the HDAC fluorescent activity assay kit (BIOMOL, Plymouth Meeting, Pa., USA) according to the manufacturer'... | US Patent US9115116 (2015) BindingDB Entry DOI: 10.7270/Q2X34W7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM175098 (US9115116, 6b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | 25 |
Academia Sinica; National Taiwan University US Patent | Assay Description The HDAC activity was performed using the HDAC fluorescent activity assay kit (BIOMOL, Plymouth Meeting, Pa., USA) according to the manufacturer'... | US Patent US9115116 (2015) BindingDB Entry DOI: 10.7270/Q2X34W7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50432563 (CHEMBL2347011 | US9115116, 8b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | 25 |
Academia Sinica; National Taiwan University US Patent | Assay Description The HDAC activity was performed using the HDAC fluorescent activity assay kit (BIOMOL, Plymouth Meeting, Pa., USA) according to the manufacturer'... | US Patent US9115116 (2015) BindingDB Entry DOI: 10.7270/Q2X34W7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM175097 (US9115116, 6a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | 25 |
Academia Sinica; National Taiwan University US Patent | Assay Description The HDAC activity was performed using the HDAC fluorescent activity assay kit (BIOMOL, Plymouth Meeting, Pa., USA) according to the manufacturer'... | US Patent US9115116 (2015) BindingDB Entry DOI: 10.7270/Q2X34W7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50432564 (CHEMBL2347010 | US9115116, 8a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | 25 |
Academia Sinica; National Taiwan University US Patent | Assay Description The HDAC activity was performed using the HDAC fluorescent activity assay kit (BIOMOL, Plymouth Meeting, Pa., USA) according to the manufacturer'... | US Patent US9115116 (2015) BindingDB Entry DOI: 10.7270/Q2X34W7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50432562 (CHEMBL2347005 | US9115116, 12a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | 25 |
Academia Sinica; National Taiwan University US Patent | Assay Description The HDAC activity was performed using the HDAC fluorescent activity assay kit (BIOMOL, Plymouth Meeting, Pa., USA) according to the manufacturer'... | US Patent US9115116 (2015) BindingDB Entry DOI: 10.7270/Q2X34W7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM175097 (US9115116, 6a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 159 | n/a | n/a | n/a | n/a | n/a | 25 |
Academia Sinica; National Taiwan University US Patent | Assay Description The HDAC activity was performed using the HDAC fluorescent activity assay kit (BIOMOL, Plymouth Meeting, Pa., USA) according to the manufacturer'... | US Patent US9115116 (2015) BindingDB Entry DOI: 10.7270/Q2X34W7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50432564 (CHEMBL2347010 | US9115116, 8a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 159 | n/a | n/a | n/a | n/a | n/a | 25 |
Academia Sinica; National Taiwan University US Patent | Assay Description The HDAC activity was performed using the HDAC fluorescent activity assay kit (BIOMOL, Plymouth Meeting, Pa., USA) according to the manufacturer'... | US Patent US9115116 (2015) BindingDB Entry DOI: 10.7270/Q2X34W7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50432564 (CHEMBL2347010 | US9115116, 8a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 463 | n/a | n/a | n/a | n/a | n/a | 25 |
Academia Sinica; National Taiwan University US Patent | Assay Description The HDAC activity was performed using the HDAC fluorescent activity assay kit (BIOMOL, Plymouth Meeting, Pa., USA) according to the manufacturer'... | US Patent US9115116 (2015) BindingDB Entry DOI: 10.7270/Q2X34W7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM175097 (US9115116, 6a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 463 | n/a | n/a | n/a | n/a | n/a | 25 |
Academia Sinica; National Taiwan University US Patent | Assay Description The HDAC activity was performed using the HDAC fluorescent activity assay kit (BIOMOL, Plymouth Meeting, Pa., USA) according to the manufacturer'... | US Patent US9115116 (2015) BindingDB Entry DOI: 10.7270/Q2X34W7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50432562 (CHEMBL2347005 | US9115116, 12a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 658 | n/a | n/a | n/a | n/a | n/a | 25 |
Academia Sinica; National Taiwan University US Patent | Assay Description The HDAC activity was performed using the HDAC fluorescent activity assay kit (BIOMOL, Plymouth Meeting, Pa., USA) according to the manufacturer'... | US Patent US9115116 (2015) BindingDB Entry DOI: 10.7270/Q2X34W7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM175098 (US9115116, 6b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 882 | n/a | n/a | n/a | n/a | n/a | 25 |
Academia Sinica; National Taiwan University US Patent | Assay Description The HDAC activity was performed using the HDAC fluorescent activity assay kit (BIOMOL, Plymouth Meeting, Pa., USA) according to the manufacturer'... | US Patent US9115116 (2015) BindingDB Entry DOI: 10.7270/Q2X34W7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50432563 (CHEMBL2347011 | US9115116, 8b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 882 | n/a | n/a | n/a | n/a | n/a | 25 |
Academia Sinica; National Taiwan University US Patent | Assay Description The HDAC activity was performed using the HDAC fluorescent activity assay kit (BIOMOL, Plymouth Meeting, Pa., USA) according to the manufacturer'... | US Patent US9115116 (2015) BindingDB Entry DOI: 10.7270/Q2X34W7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM34168 (LOVASTATIN | MLS000069585 | SMR000058779 | US91151...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
Academia Sinica; National Taiwan University US Patent | Assay Description The HDAC activity was performed using the HDAC fluorescent activity assay kit (BIOMOL, Plymouth Meeting, Pa., USA) according to the manufacturer'... | US Patent US9115116 (2015) BindingDB Entry DOI: 10.7270/Q2X34W7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM34168 (LOVASTATIN | MLS000069585 | SMR000058779 | US91151...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
Academia Sinica; National Taiwan University US Patent | Assay Description The HDAC activity was performed using the HDAC fluorescent activity assay kit (BIOMOL, Plymouth Meeting, Pa., USA) according to the manufacturer'... | US Patent US9115116 (2015) BindingDB Entry DOI: 10.7270/Q2X34W7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM34168 (LOVASTATIN | MLS000069585 | SMR000058779 | US91151...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank US Patent | n/a | n/a | 2.59E+4 | n/a | n/a | n/a | n/a | n/a | 25 |
Academia Sinica; National Taiwan University US Patent | Assay Description The HDAC activity was performed using the HDAC fluorescent activity assay kit (BIOMOL, Plymouth Meeting, Pa., USA) according to the manufacturer'... | US Patent US9115116 (2015) BindingDB Entry DOI: 10.7270/Q2X34W7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||