

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200783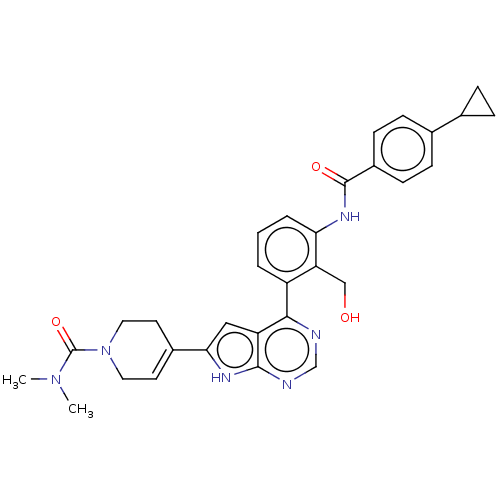 (US9233111, 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200756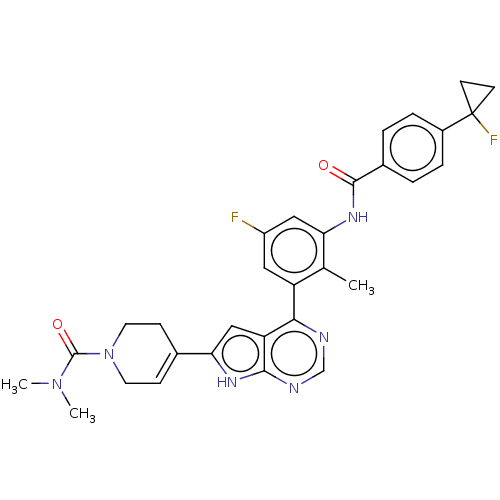 (US9233111, 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200888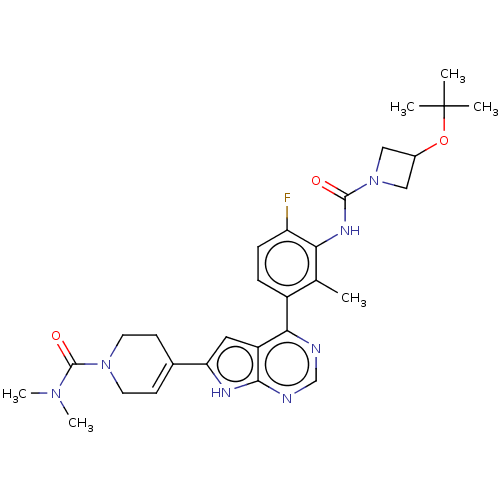 (US9233111, 63) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200778 (US9233111, 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200802 (US9233111, 25) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200887 (US9233111, 62) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200768 (US9233111, 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200766 (US9233111, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200860 (US9233111, 47) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200881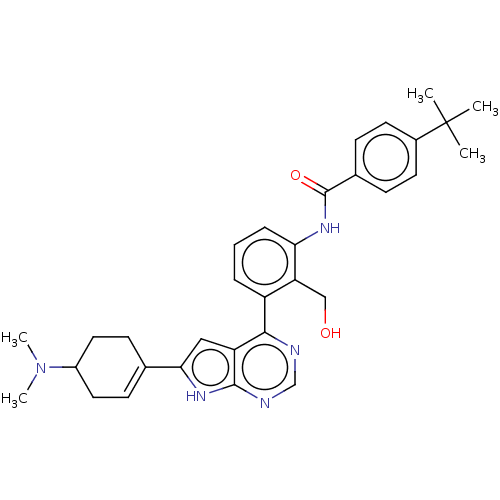 (US9233111, 56) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200884 (US9233111, 59) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200767 (US9233111, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200769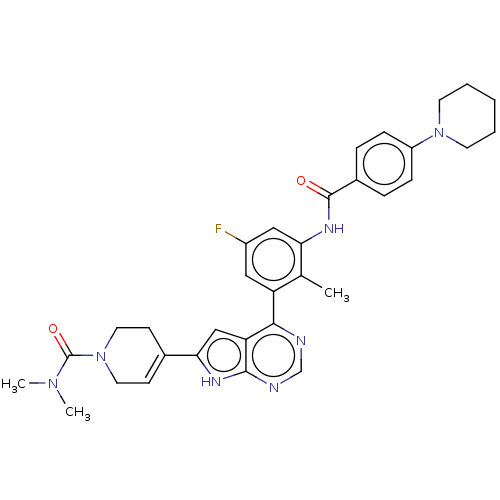 (US9233111, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200817 (US9233111, 31) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200770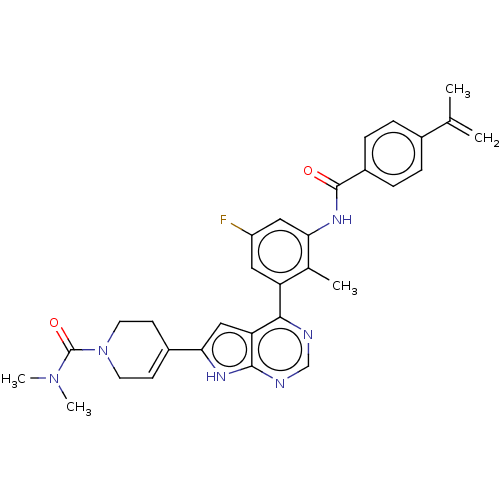 (US9233111, 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200825 (US9233111, 36) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200878 (US9233111, 55) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200775 (US9233111, 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200774 (US9233111, 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200764 (US9233111, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200819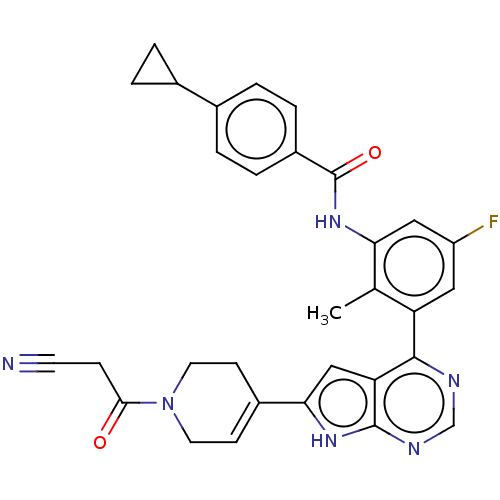 (US9233111, 33) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200773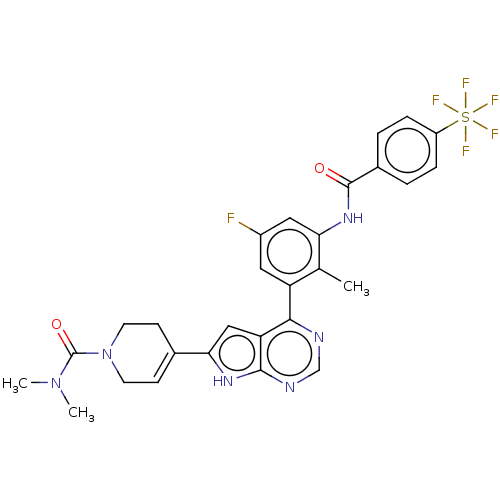 (US9233111, 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200765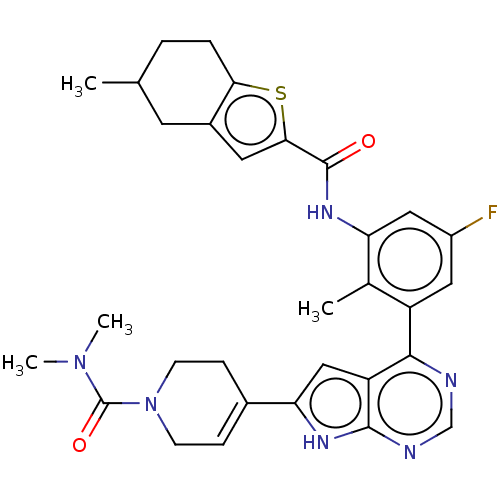 (US9233111, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200771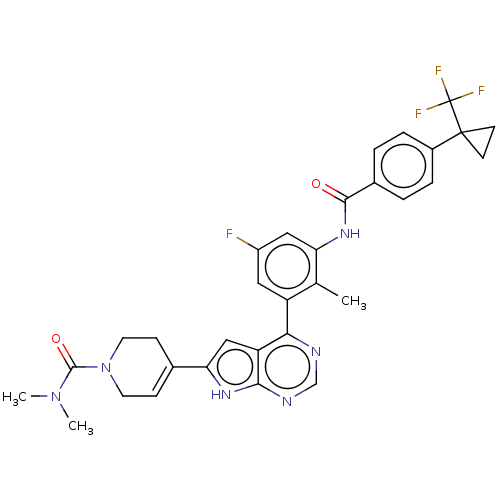 (US9233111, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200816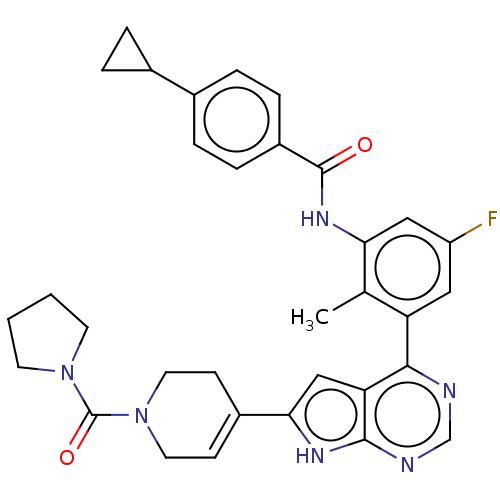 (US9233111, 30) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200784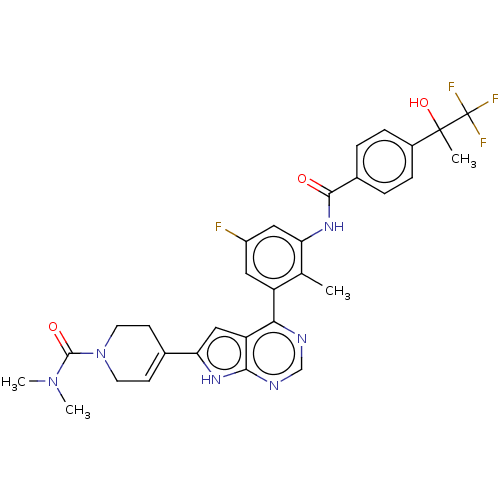 (US9233111, 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200789 (US9233111, 21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200786 (US9233111, 20) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200886 (US9233111, 61) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200818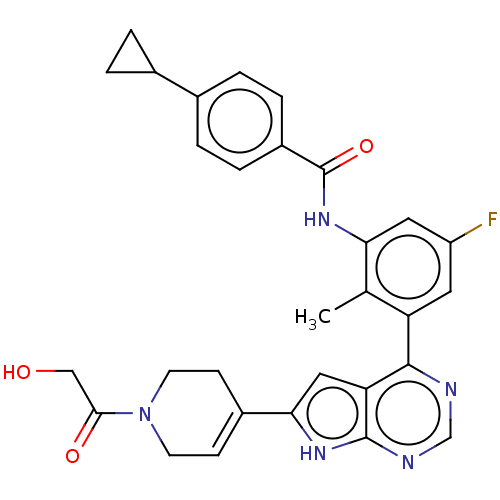 (US9233111, 32) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200882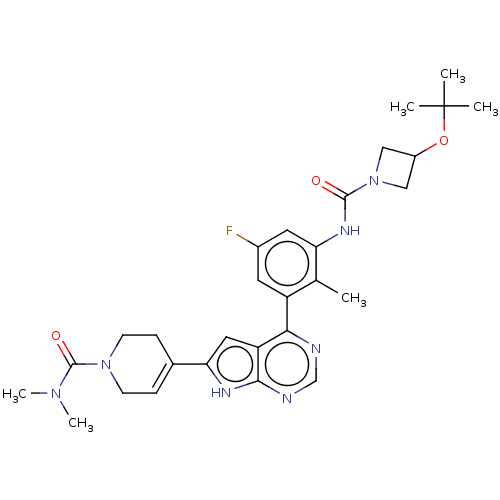 (US9233111, 57) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200866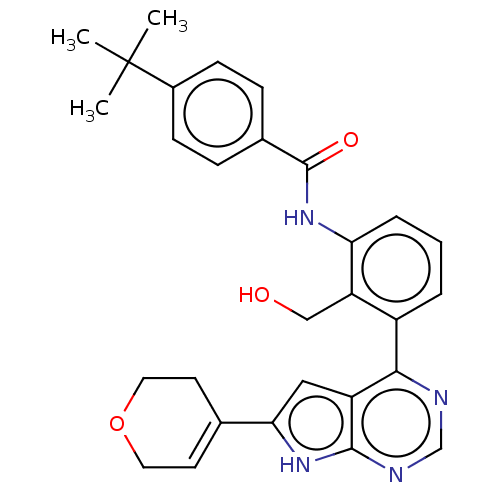 (US9233111, 48) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200901 (US9233111, 67) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200790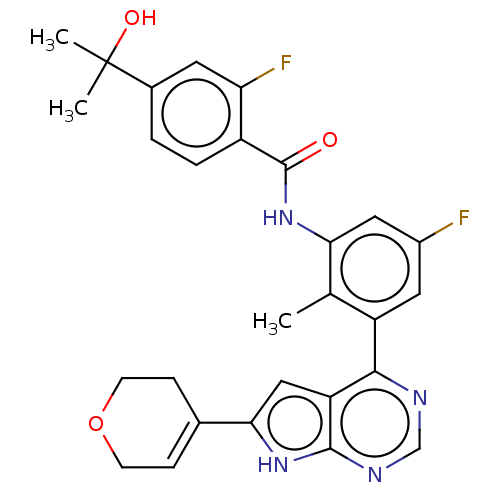 (US9233111, 22) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200885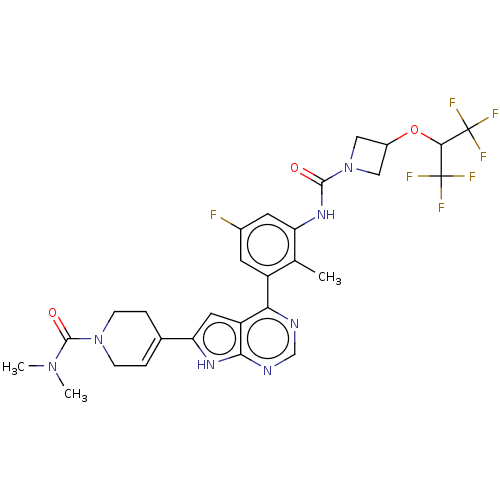 (US9233111, 60) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200854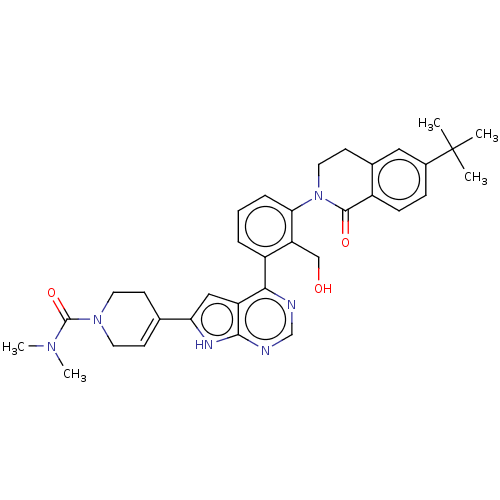 (US9233111, 45) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200897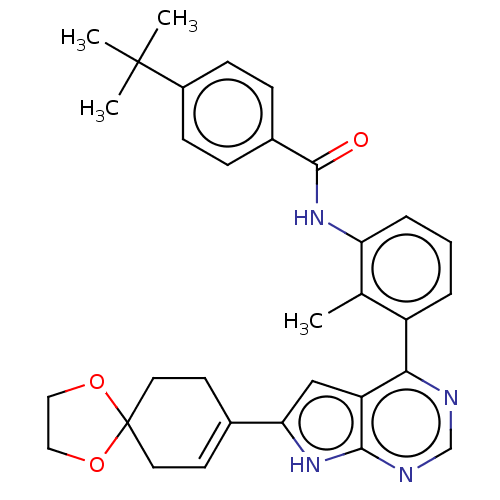 (US9233111, 65) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200898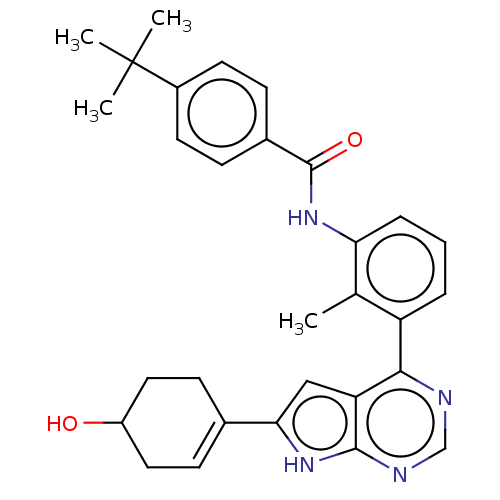 (US9233111, 66) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200794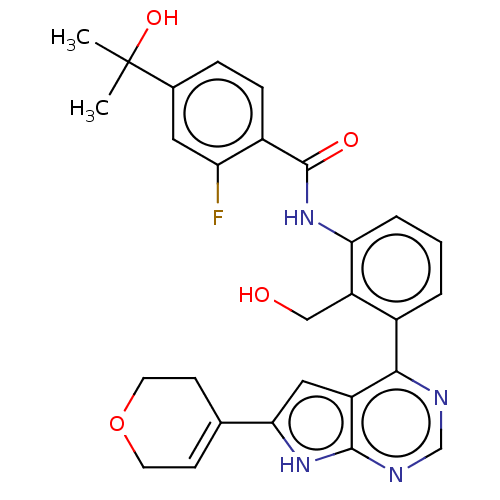 (US9233111, 23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200811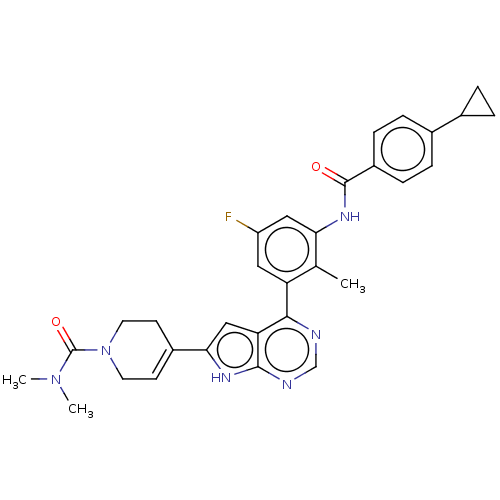 (US9233111, 29) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200838 (US9233111, 39) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200824 (US9233111, 35) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200772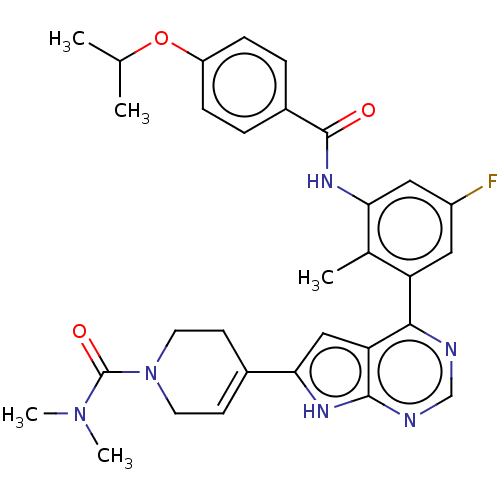 (US9233111, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200891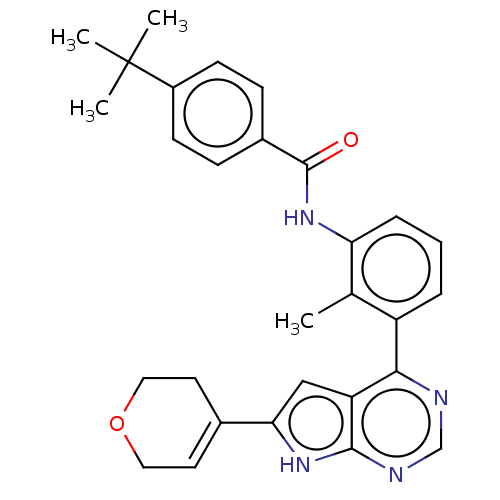 (US9233111, 64) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200776 (US9233111, 14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200883 (US9233111, 58) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200851 (US9233111, 44) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200777 (US9233111, 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200857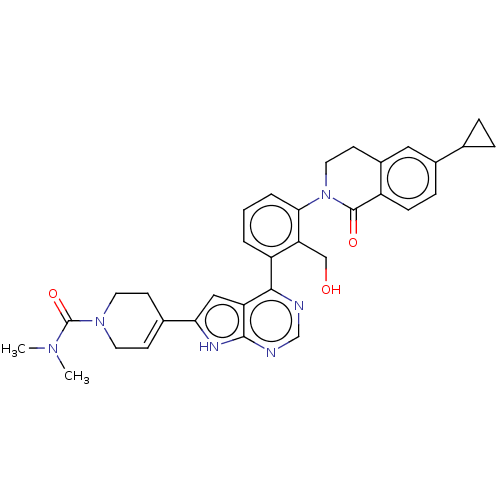 (US9233111, 46) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200844 (US9233111, 40) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200835 (US9233111, 38) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | 4 |
NOVARTIS AG US Patent | Assay Description Alternatively, the present compounds might also be assessed for their capacity to inhibit Btk-dependent FcG receptor-induced IL-8 secretion in human ... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200785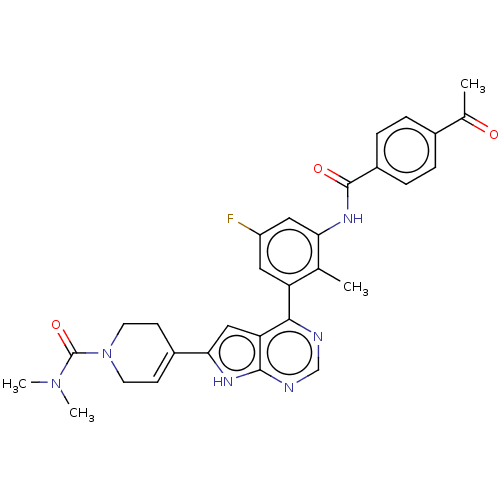 (US9233111, 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200848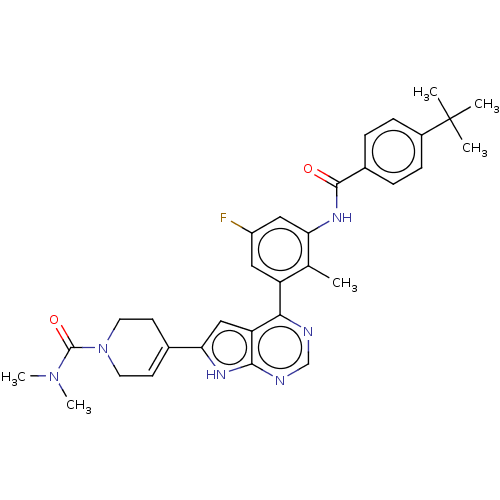 (US9233111, 41) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200798 (US9233111, 24) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200875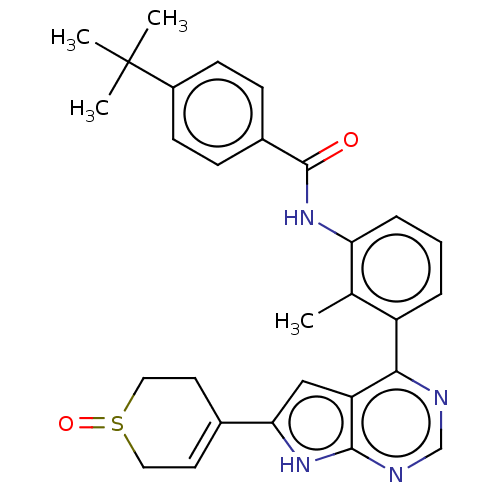 (US9233111, 52) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200877 (US9233111, 54) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200826 (US9233111, 37) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200835 (US9233111, 38) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200765 (US9233111, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | 4 |
NOVARTIS AG US Patent | Assay Description Alternatively, the present compounds might also be assessed for their capacity to inhibit Btk-dependent FcG receptor-induced IL-8 secretion in human ... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200849 (US9233111, 42) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200820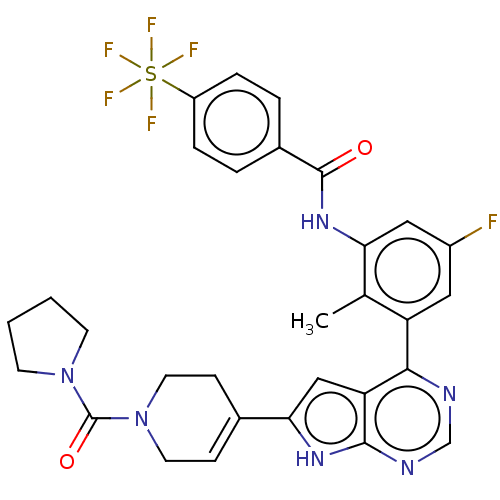 (US9233111, 34) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 34 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200803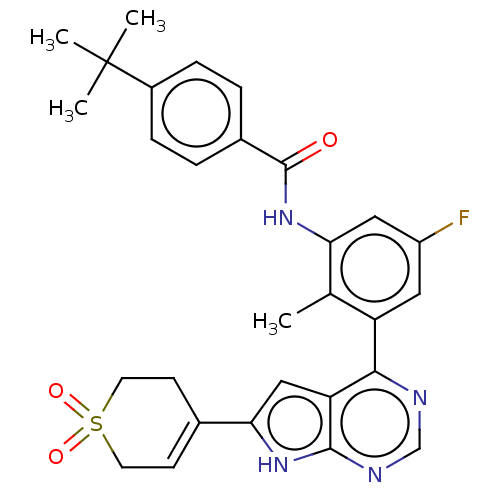 (US9233111, 26) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200808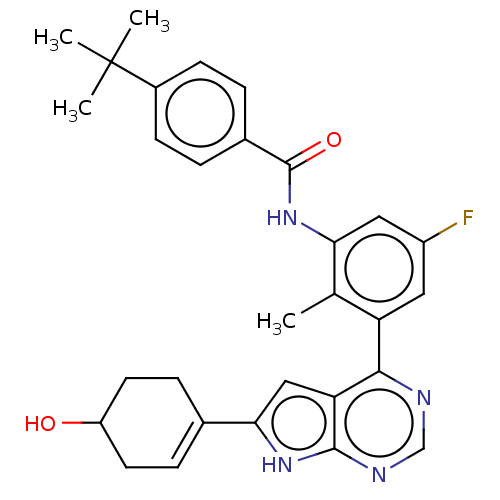 (US9233111, 28) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 46 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200868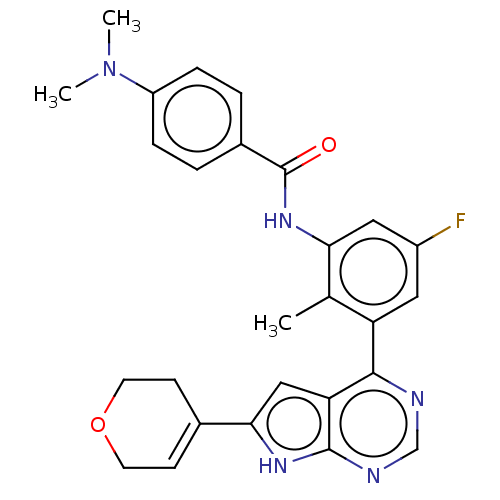 (US9233111, 50) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 47 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200872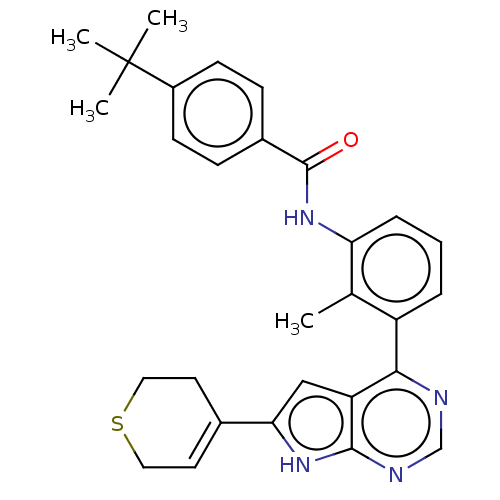 (US9233111, 51) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 47 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200876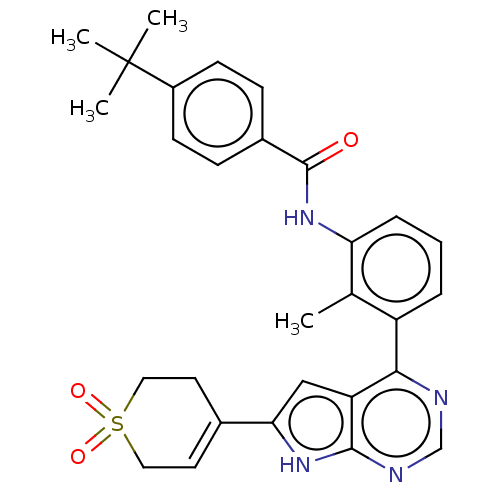 (US9233111, 53) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 64 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200778 (US9233111, 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | 4 |
NOVARTIS AG US Patent | Assay Description Alternatively, the present compounds might also be assessed for their capacity to inhibit Btk-dependent FcG receptor-induced IL-8 secretion in human ... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200887 (US9233111, 62) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | 4 |
NOVARTIS AG US Patent | Assay Description Alternatively, the present compounds might also be assessed for their capacity to inhibit Btk-dependent FcG receptor-induced IL-8 secretion in human ... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200798 (US9233111, 24) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | 4 |
NOVARTIS AG US Patent | Assay Description Alternatively, the present compounds might also be assessed for their capacity to inhibit Btk-dependent FcG receptor-induced IL-8 secretion in human ... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200867 (US9233111, 49) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 99 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200804 (US9233111, 27) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 110 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM200850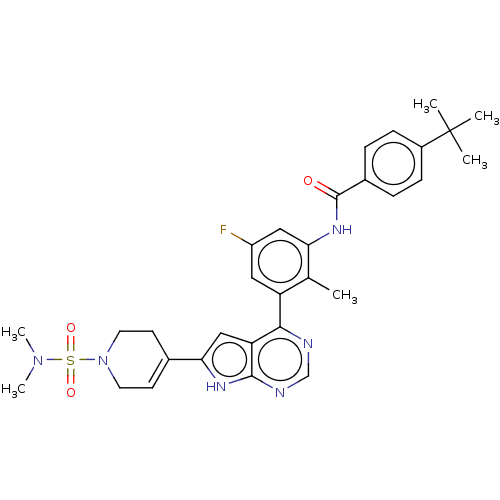 (US9233111, 43) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9233111 (2016) BindingDB Entry DOI: 10.7270/Q2ZS2V9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||