

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM223008 (US10034939, Example 10 | US10967060, Example 10 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 6.5 | 25 |
Incyte Corporation; Incyte Holdings Corporation US Patent | Assay Description Human indoleamine 2,3-dioxygenasae (IDO) with an N-terminal His tag was expressed in E. coli and purified to homogeneity. IDO catalyzes the oxidative... | US Patent US9320732 (2016) BindingDB Entry DOI: 10.7270/Q26M35Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM223009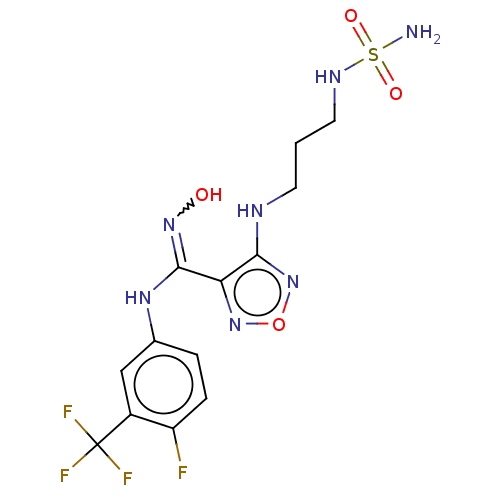 (US10034939, Example 11 | US10967060, Example 11 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 6.5 | 25 |
Incyte Corporation; Incyte Holdings Corporation US Patent | Assay Description Human indoleamine 2,3-dioxygenasae (IDO) with an N-terminal His tag was expressed in E. coli and purified to homogeneity. IDO catalyzes the oxidative... | US Patent US9320732 (2016) BindingDB Entry DOI: 10.7270/Q26M35Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM223001 (US10034939, Example 3 | US10967060, Example 3 | US...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 6.5 | 25 |
Incyte Corporation; Incyte Holdings Corporation US Patent | Assay Description Human indoleamine 2,3-dioxygenasae (IDO) with an N-terminal His tag was expressed in E. coli and purified to homogeneity. IDO catalyzes the oxidative... | US Patent US9320732 (2016) BindingDB Entry DOI: 10.7270/Q26M35Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM223007 (US10034939, Example 9 | US10967060, Example 9 | US...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 6.5 | 25 |
Incyte Corporation; Incyte Holdings Corporation US Patent | Assay Description Human indoleamine 2,3-dioxygenasae (IDO) with an N-terminal His tag was expressed in E. coli and purified to homogeneity. IDO catalyzes the oxidative... | US Patent US9320732 (2016) BindingDB Entry DOI: 10.7270/Q26M35Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM223002 (US10034939, Example 4 | US10967060, Example 4 | US...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 6.5 | 25 |
Incyte Corporation; Incyte Holdings Corporation US Patent | Assay Description Human indoleamine 2,3-dioxygenasae (IDO) with an N-terminal His tag was expressed in E. coli and purified to homogeneity. IDO catalyzes the oxidative... | US Patent US9320732 (2016) BindingDB Entry DOI: 10.7270/Q26M35Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM223010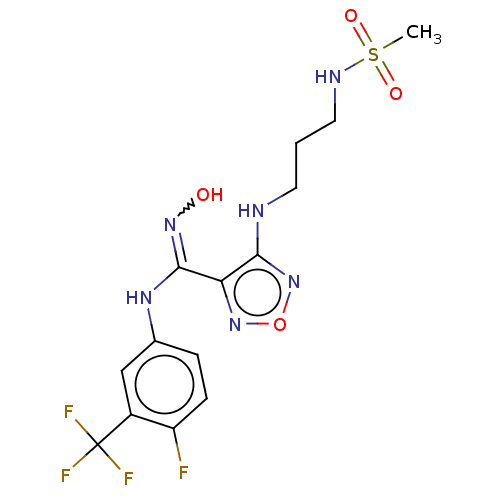 (US10034939, Example 12 | US10967060, Example 12 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 6.5 | 25 |
Incyte Corporation; Incyte Holdings Corporation US Patent | Assay Description Human indoleamine 2,3-dioxygenasae (IDO) with an N-terminal His tag was expressed in E. coli and purified to homogeneity. IDO catalyzes the oxidative... | US Patent US9320732 (2016) BindingDB Entry DOI: 10.7270/Q26M35Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM223014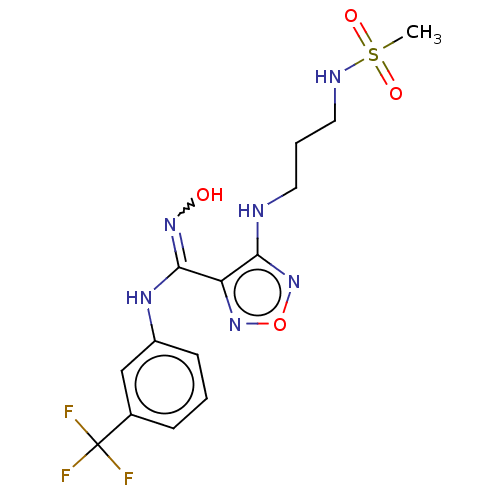 (US10034939, Example 16 | US10967060, Example 16 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | 6.5 | 25 |
Incyte Corporation; Incyte Holdings Corporation US Patent | Assay Description Human indoleamine 2,3-dioxygenasae (IDO) with an N-terminal His tag was expressed in E. coli and purified to homogeneity. IDO catalyzes the oxidative... | US Patent US9320732 (2016) BindingDB Entry DOI: 10.7270/Q26M35Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM223013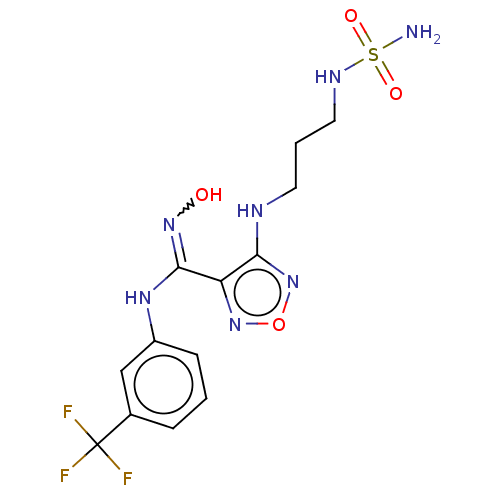 (US10034939, Example 15 | US10967060, Example 15 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | 6.5 | 25 |
Incyte Corporation; Incyte Holdings Corporation US Patent | Assay Description Human indoleamine 2,3-dioxygenasae (IDO) with an N-terminal His tag was expressed in E. coli and purified to homogeneity. IDO catalyzes the oxidative... | US Patent US9320732 (2016) BindingDB Entry DOI: 10.7270/Q26M35Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM223012 (US10034864, Example 14 | US10967060, Example 14 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | 6.5 | 25 |
Incyte Corporation; Incyte Holdings Corporation US Patent | Assay Description Human indoleamine 2,3-dioxygenasae (IDO) with an N-terminal His tag was expressed in E. coli and purified to homogeneity. IDO catalyzes the oxidative... | US Patent US9320732 (2016) BindingDB Entry DOI: 10.7270/Q26M35Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM223004 (US10034939, Example 6 | US10967060, Example 6 | US...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | 6.5 | 25 |
Incyte Corporation; Incyte Holdings Corporation US Patent | Assay Description Human indoleamine 2,3-dioxygenasae (IDO) with an N-terminal His tag was expressed in E. coli and purified to homogeneity. IDO catalyzes the oxidative... | US Patent US9320732 (2016) BindingDB Entry DOI: 10.7270/Q26M35Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM223003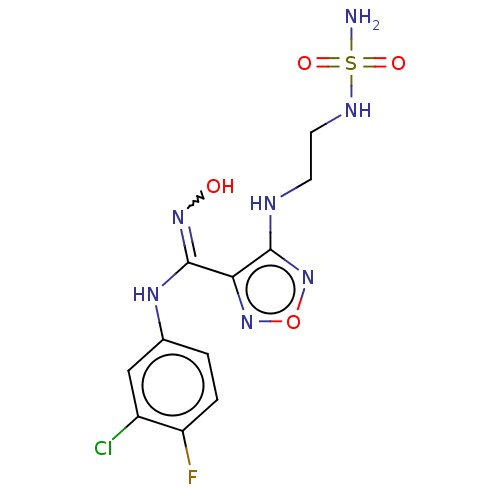 (US10034939, Example 5 | US10967060, Example 5 | US...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | 6.5 | 25 |
Incyte Corporation; Incyte Holdings Corporation US Patent | Assay Description Human indoleamine 2,3-dioxygenasae (IDO) with an N-terminal His tag was expressed in E. coli and purified to homogeneity. IDO catalyzes the oxidative... | US Patent US9320732 (2016) BindingDB Entry DOI: 10.7270/Q26M35Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM223005 (US10034939, Example 7 | US10967060, Example 7 | US...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | 6.5 | 25 |
Incyte Corporation; Incyte Holdings Corporation US Patent | Assay Description Human indoleamine 2,3-dioxygenasae (IDO) with an N-terminal His tag was expressed in E. coli and purified to homogeneity. IDO catalyzes the oxidative... | US Patent US9320732 (2016) BindingDB Entry DOI: 10.7270/Q26M35Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM223000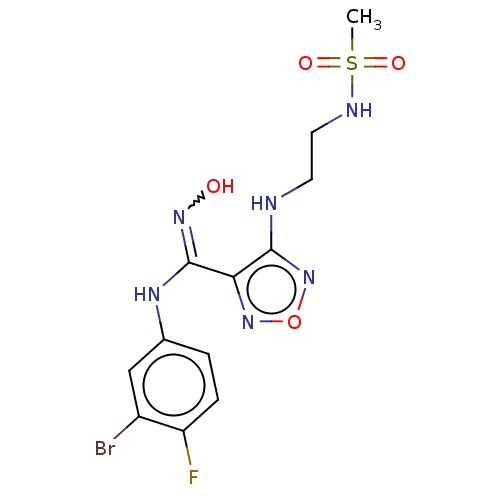 (US10034939, Example 2 | US10967060, Example 2 | US...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | 6.5 | 25 |
Incyte Corporation; Incyte Holdings Corporation US Patent | Assay Description Human indoleamine 2,3-dioxygenasae (IDO) with an N-terminal His tag was expressed in E. coli and purified to homogeneity. IDO catalyzes the oxidative... | US Patent US9320732 (2016) BindingDB Entry DOI: 10.7270/Q26M35Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM223006 (US10034939, Example 8 | US10967060, Example 8 | US...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | 6.5 | 25 |
Incyte Corporation; Incyte Holdings Corporation US Patent | Assay Description Human indoleamine 2,3-dioxygenasae (IDO) with an N-terminal His tag was expressed in E. coli and purified to homogeneity. IDO catalyzes the oxidative... | US Patent US9320732 (2016) BindingDB Entry DOI: 10.7270/Q26M35Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM222999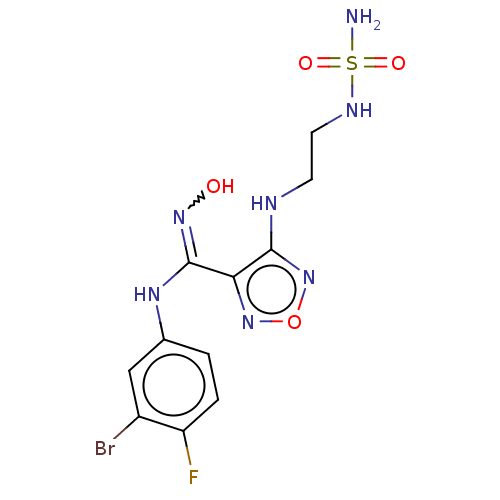 (US10034864, Example 1 | US10967060, Example 1 | US...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | <200 | n/a | n/a | n/a | n/a | 6.5 | 25 |
Incyte Corporation; Incyte Holdings Corporation US Patent | Assay Description Human indoleamine 2,3-dioxygenasae (IDO) with an N-terminal His tag was expressed in E. coli and purified to homogeneity. IDO catalyzes the oxidative... | US Patent US9320732 (2016) BindingDB Entry DOI: 10.7270/Q26M35Q9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM223017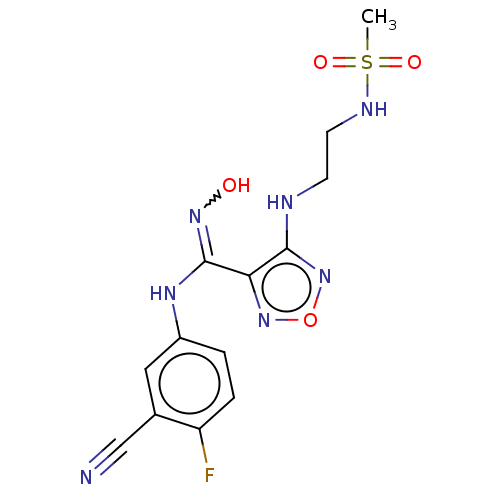 (US10034939, Example 19 | US10967060, Example 19 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | 6.5 | 25 |
Incyte Corporation; Incyte Holdings Corporation US Patent | Assay Description Human indoleamine 2,3-dioxygenasae (IDO) with an N-terminal His tag was expressed in E. coli and purified to homogeneity. IDO catalyzes the oxidative... | US Patent US9320732 (2016) BindingDB Entry DOI: 10.7270/Q26M35Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM223015 (US10034939, Example 17 | US10967060, Example 17 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | 6.5 | 25 |
Incyte Corporation; Incyte Holdings Corporation US Patent | Assay Description Human indoleamine 2,3-dioxygenasae (IDO) with an N-terminal His tag was expressed in E. coli and purified to homogeneity. IDO catalyzes the oxidative... | US Patent US9320732 (2016) BindingDB Entry DOI: 10.7270/Q26M35Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM223011 (US10034939, Example 13 | US10967060, Example 13 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | 6.5 | 25 |
Incyte Corporation; Incyte Holdings Corporation US Patent | Assay Description Human indoleamine 2,3-dioxygenasae (IDO) with an N-terminal His tag was expressed in E. coli and purified to homogeneity. IDO catalyzes the oxidative... | US Patent US9320732 (2016) BindingDB Entry DOI: 10.7270/Q26M35Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM223018 (US10034939, Example 20 | US10967060, Example 20 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | 6.5 | 25 |
Incyte Corporation; Incyte Holdings Corporation US Patent | Assay Description Human indoleamine 2,3-dioxygenasae (IDO) with an N-terminal His tag was expressed in E. coli and purified to homogeneity. IDO catalyzes the oxidative... | US Patent US9320732 (2016) BindingDB Entry DOI: 10.7270/Q26M35Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM223018 (US10034939, Example 20 | US10967060, Example 20 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <750 | n/a | n/a | n/a | n/a | 6.5 | 25 |
Incyte Corporation; Incyte Holdings Corporation US Patent | Assay Description Human indoleamine 2,3-dioxygenasae (IDO) with an N-terminal His tag was expressed in E. coli and purified to homogeneity. IDO catalyzes the oxidative... | US Patent US9320732 (2016) BindingDB Entry DOI: 10.7270/Q26M35Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM223016 (US10034939, Example 18 | US10967060, Example 18 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <750 | n/a | n/a | n/a | n/a | 6.5 | 25 |
Incyte Corporation; Incyte Holdings Corporation US Patent | Assay Description Human indoleamine 2,3-dioxygenasae (IDO) with an N-terminal His tag was expressed in E. coli and purified to homogeneity. IDO catalyzes the oxidative... | US Patent US9320732 (2016) BindingDB Entry DOI: 10.7270/Q26M35Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||