

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50134248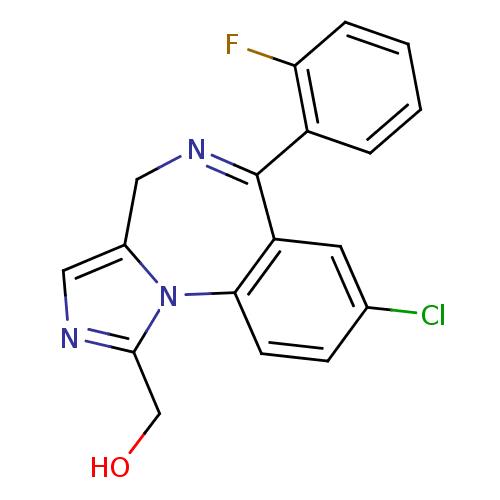 (Alpha-Hydroxy-Midazolam | CHEMBL1188 | US9333197, ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Six test compound concentrations (0.1, 0.25, 1, 2.5, 10, 25 μM in DMSO; final DMSO concentration=0.26%) are incubated with human liver microsome... | US Patent US9333197 (2016) BindingDB Entry DOI: 10.7270/Q2FT8JW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM224815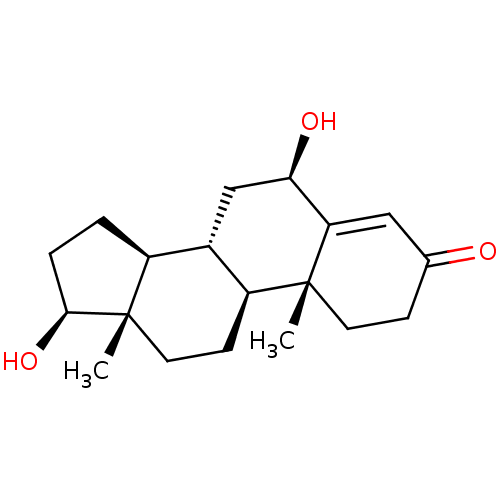 (US9333197, 6beta Hydroxytestosterone) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Six test compound concentrations (0.1, 0.25, 1, 2.5, 10, 25 μM in DMSO; final DMSO concentration=0.275%) are incubated with human liver microsom... | US Patent US9333197 (2016) BindingDB Entry DOI: 10.7270/Q2FT8JW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM224023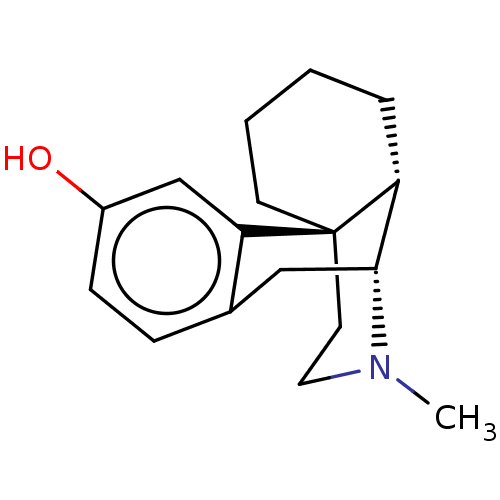 (Dextrorphan) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 1.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Six test compound concentrations (0.1, 0.25, 1, 2.5, 10, 25 μM in DMSO; final DMSO concentration=0.25%) are incubated with human liver microsome... | US Patent US9333197 (2016) BindingDB Entry DOI: 10.7270/Q2FT8JW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM224811 (US9333197, Hydroxybupropion) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Six test compound concentrations (0.1, 0.25, 1, 2.5, 10, 25 μM in DMSO; final DMSO concentration=0.3%) are incubated with human liver microsomes... | US Patent US9333197 (2016) BindingDB Entry DOI: 10.7270/Q2FT8JW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM224812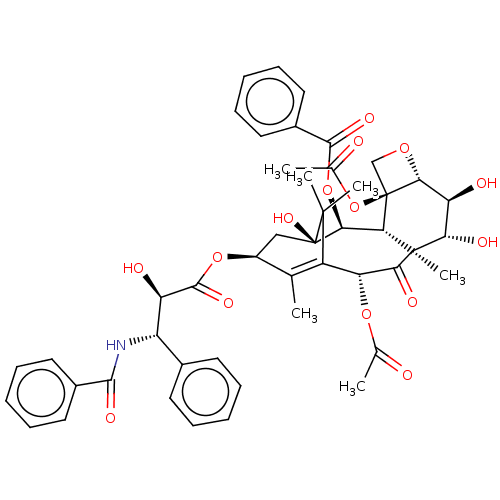 (US9333197, 6alpha-Hydroxypaclitaxel) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 2.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Six test compound concentrations (0.1, 0.25, 1, 2.5, 10, 25 μM in DMSO; final DMSO concentration=0.3%) are incubated with human liver microsomes... | US Patent US9333197 (2016) BindingDB Entry DOI: 10.7270/Q2FT8JW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM224814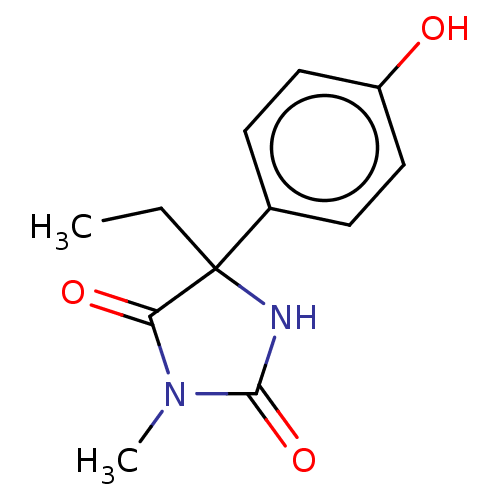 (US9333197, 4-Hydroxymephenytoin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Six test compound concentrations (0.1, 0.25, 1, 2.5, 10, 25 μM in DMSO; final DMSO concentration=0.25%) are incubated with human liver microsome... | US Patent US9333197 (2016) BindingDB Entry DOI: 10.7270/Q2FT8JW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM224813 (US9333197, 4-Hydroxytolbutamide) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Six test compound concentrations (0.1, 0.25, 1, 2.5, 10, 25 μM in DMSO; final DMSO concentration=0.25%) are incubated with human liver microsome... | US Patent US9333197 (2016) BindingDB Entry DOI: 10.7270/Q2FT8JW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM26197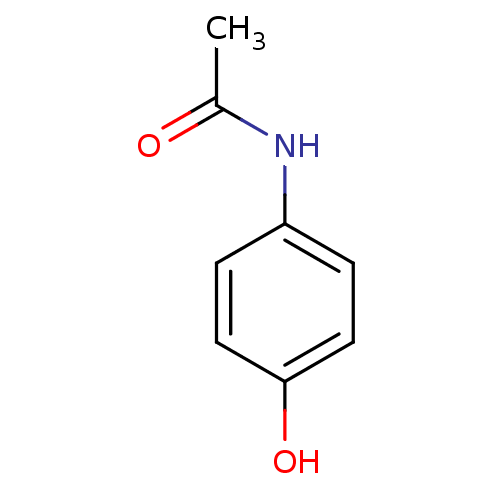 (CHEMBL112 | N-(4-hydroxyphenyl)acetamide | Norco |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | DrugBank US Patent | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Six test compound concentrations (0.1, 0.25, 1, 2.5, 10, 25 μM in DMSO; final DMSO concentration=0.3%) are incubated with human liver microsomes... | US Patent US9333197 (2016) BindingDB Entry DOI: 10.7270/Q2FT8JW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM195591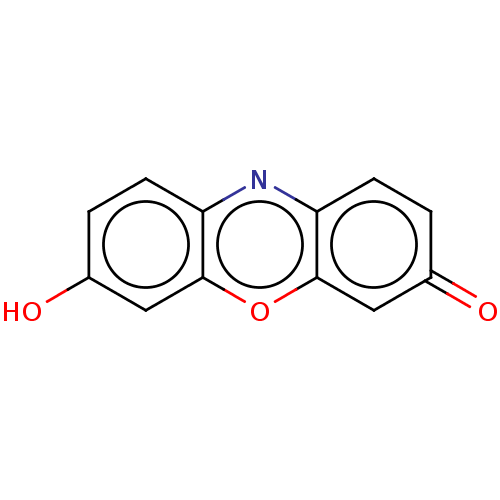 (US9216974,Resorufin | US9333197, Resorufin) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Six test compound concentrations (0.1, 0.25, 1, 2.5, 10, 25 μM in DMSO; final DMSO concentration=0.3%) are incubated with human liver microsomes... | US Patent US9333197 (2016) BindingDB Entry DOI: 10.7270/Q2FT8JW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||