

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM233390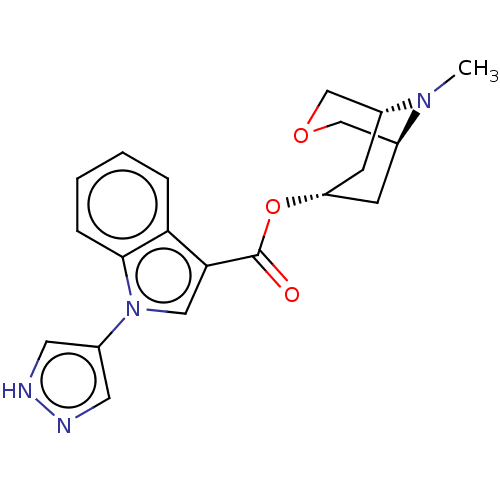 (US9346829, 29) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.462 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Phamaceutical Company Limited US Patent | Assay Description The 5-HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux ... | US Patent US9346829 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM233364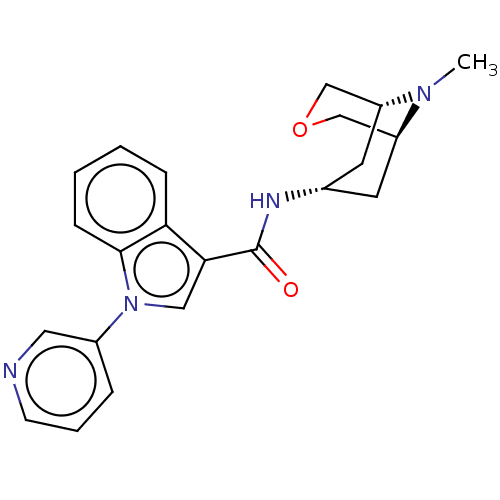 (US9346829, 2 | US9346829, 6) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Phamaceutical Company Limited US Patent | Assay Description The 5-HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux ... | US Patent US9346829 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM233373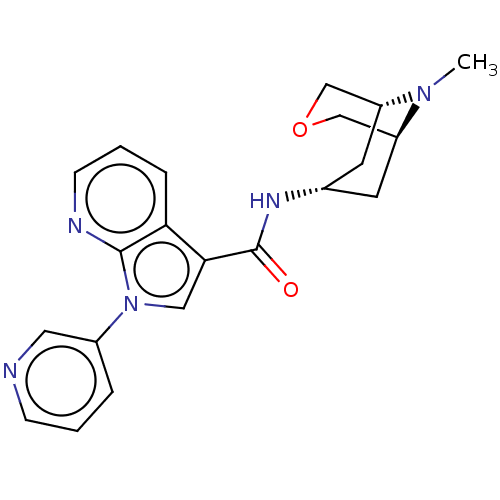 (US9346829, 11) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Phamaceutical Company Limited US Patent | Assay Description The 5-HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux ... | US Patent US9346829 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM233367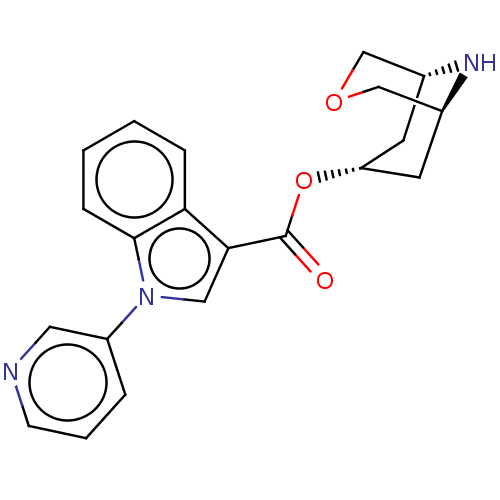 (US9346829, 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Phamaceutical Company Limited US Patent | Assay Description The 5-HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux ... | US Patent US9346829 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM233384 (US9346829, 22) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Phamaceutical Company Limited US Patent | Assay Description The 5-HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux ... | US Patent US9346829 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM233372 (US9346829, 10) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Phamaceutical Company Limited US Patent | Assay Description The 5-HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux ... | US Patent US9346829 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM233377 (US9346829, 15) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.662 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Phamaceutical Company Limited US Patent | Assay Description The 5-HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux ... | US Patent US9346829 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM233371 (US9346829, 9) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Phamaceutical Company Limited US Patent | Assay Description The 5-HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux ... | US Patent US9346829 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM233366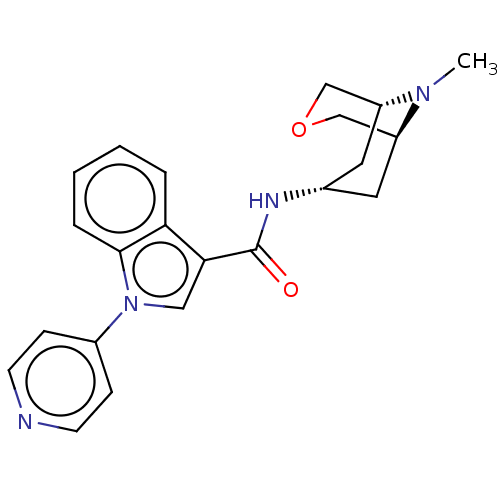 (US9346829, 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Phamaceutical Company Limited US Patent | Assay Description The 5-HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux ... | US Patent US9346829 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM233375 (US9346829, 13) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Phamaceutical Company Limited US Patent | Assay Description The 5-HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux ... | US Patent US9346829 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM233391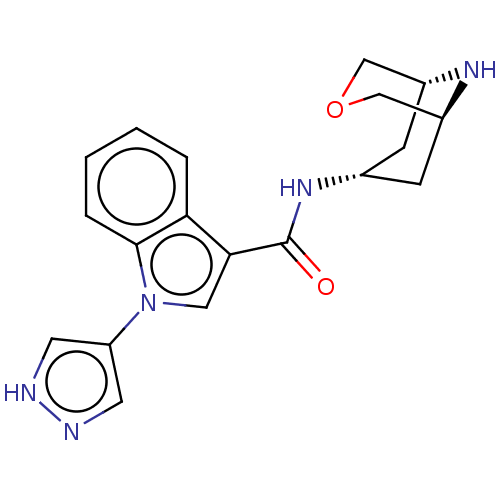 (US9346829, 30) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.745 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Phamaceutical Company Limited US Patent | Assay Description The 5-HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux ... | US Patent US9346829 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM233379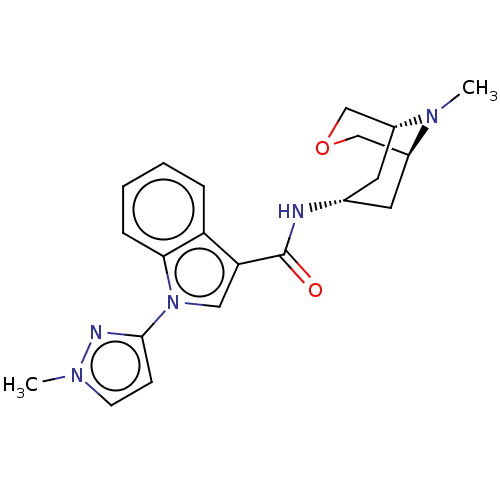 (US9346829, 17) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Phamaceutical Company Limited US Patent | Assay Description The 5-HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux ... | US Patent US9346829 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM233365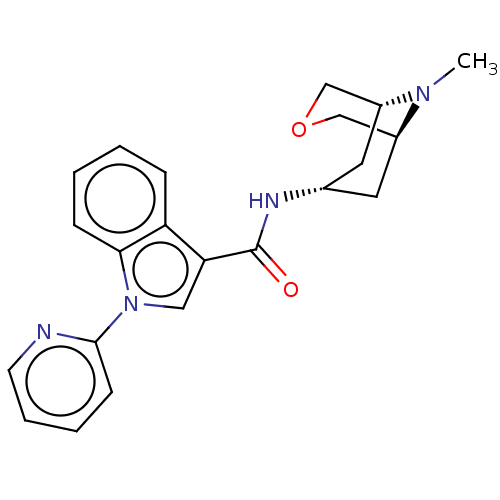 (US9346829, 3 | US9346829, 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Phamaceutical Company Limited US Patent | Assay Description The 5-HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux ... | US Patent US9346829 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM233374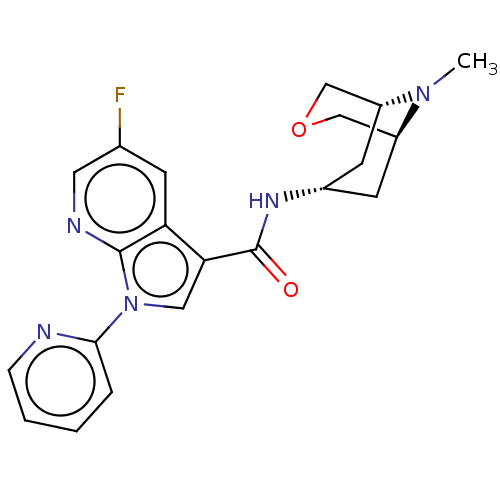 (US9346829, 12) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.882 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Phamaceutical Company Limited US Patent | Assay Description The 5-HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux ... | US Patent US9346829 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM233363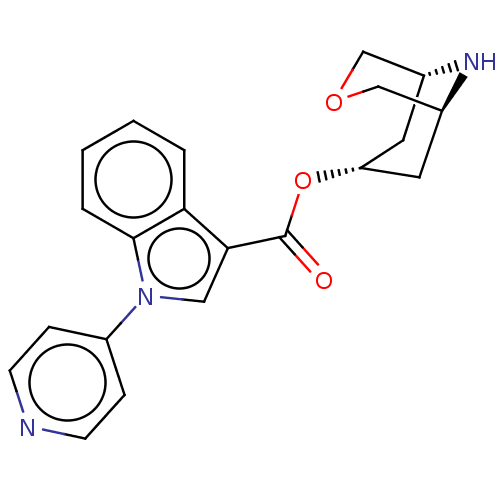 (US9346829, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Phamaceutical Company Limited US Patent | Assay Description The 5-HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux ... | US Patent US9346829 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM233365 (US9346829, 3 | US9346829, 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Phamaceutical Company Limited US Patent | Assay Description The 5-HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux ... | US Patent US9346829 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM233385 (US9346829, 23) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.01 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Phamaceutical Company Limited US Patent | Assay Description The 5-HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux ... | US Patent US9346829 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM233364 (US9346829, 2 | US9346829, 6) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Phamaceutical Company Limited US Patent | Assay Description The 5-HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux ... | US Patent US9346829 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM233383 (US9346829, 21) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Phamaceutical Company Limited US Patent | Assay Description The 5-HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux ... | US Patent US9346829 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM233387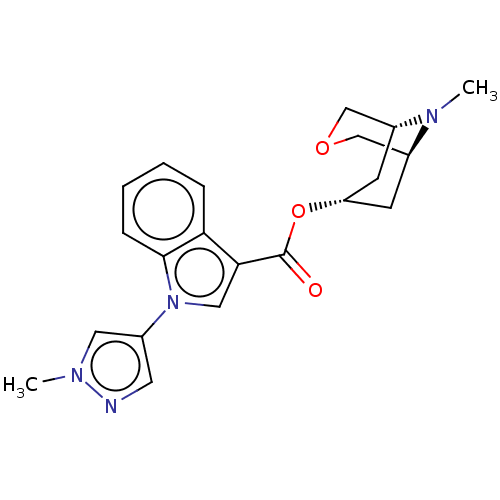 (US9346829, 26) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Phamaceutical Company Limited US Patent | Assay Description The 5-HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux ... | US Patent US9346829 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM233376 (US9346829, 14) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.34 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Phamaceutical Company Limited US Patent | Assay Description The 5-HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux ... | US Patent US9346829 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM233393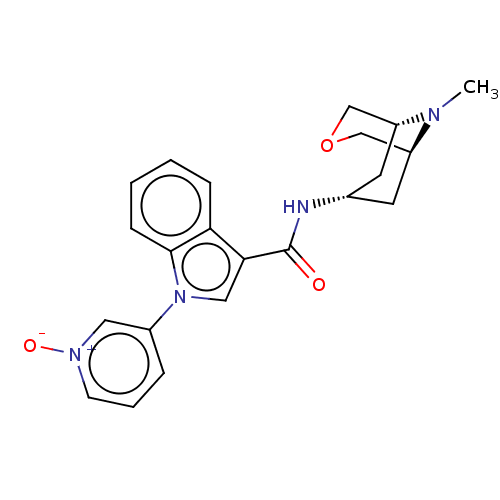 (US9346829, 32) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.36 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Phamaceutical Company Limited US Patent | Assay Description The 5-HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux ... | US Patent US9346829 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM233386 (US9346829, 25) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.39 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Phamaceutical Company Limited US Patent | Assay Description The 5-HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux ... | US Patent US9346829 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM233388 (US9346829, 27) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.44 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Phamaceutical Company Limited US Patent | Assay Description The 5-HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux ... | US Patent US9346829 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM233382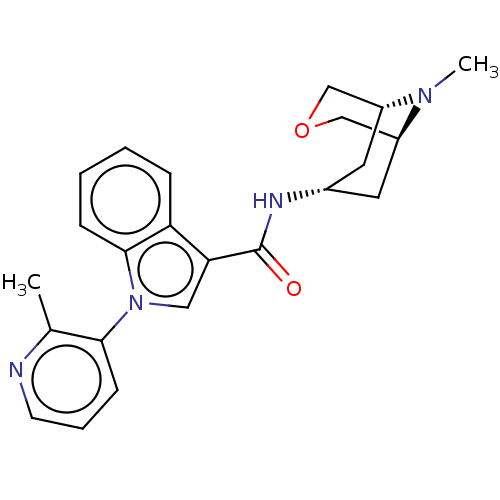 (US9346829, 20) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Phamaceutical Company Limited US Patent | Assay Description The 5-HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux ... | US Patent US9346829 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM233380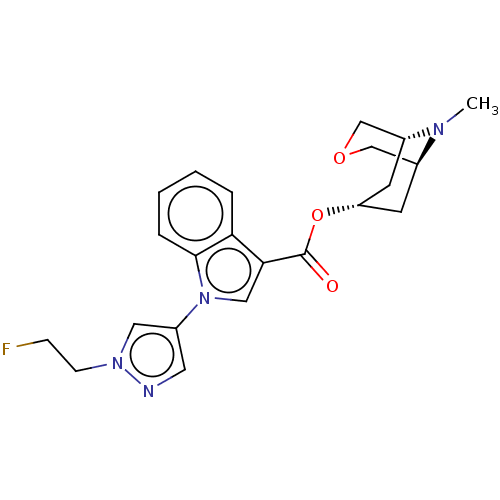 (US9346829, 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.01 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Phamaceutical Company Limited US Patent | Assay Description The 5-HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux ... | US Patent US9346829 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM233389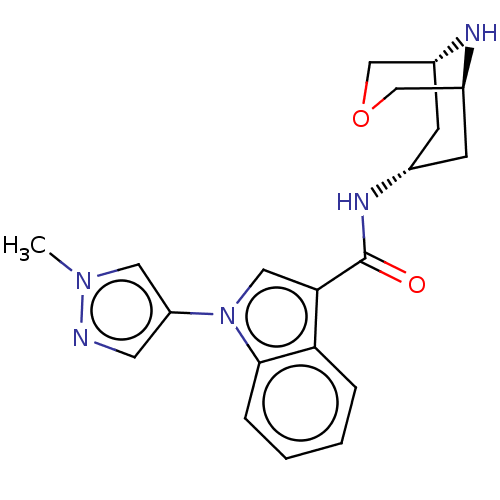 (US9346829, 28) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.04 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Phamaceutical Company Limited US Patent | Assay Description The 5-HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux ... | US Patent US9346829 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM233378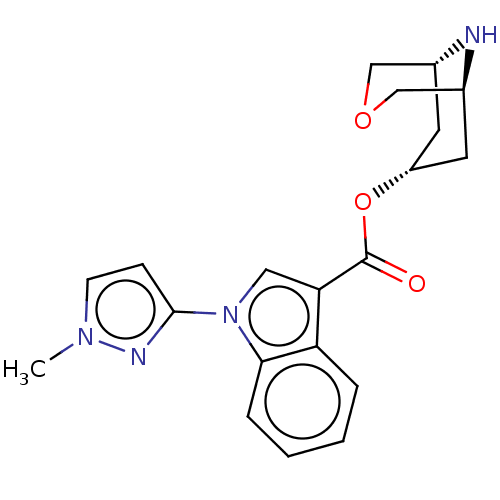 (US9346829, 16) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Phamaceutical Company Limited US Patent | Assay Description The 5-HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux ... | US Patent US9346829 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM233369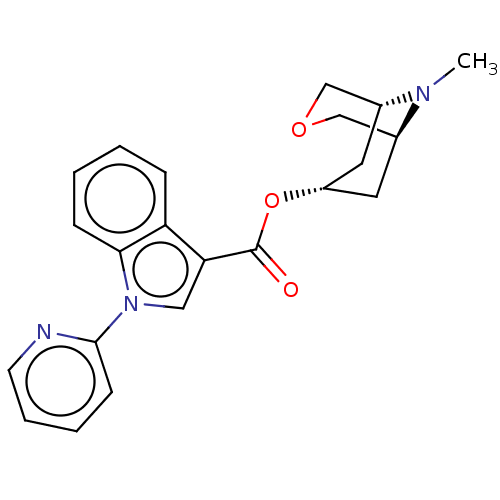 (US9346829, 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.86 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Phamaceutical Company Limited US Patent | Assay Description The 5-HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux ... | US Patent US9346829 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM233381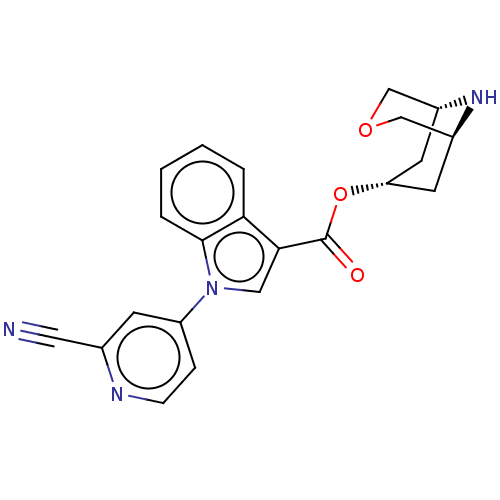 (US9346829, 19) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.68 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Phamaceutical Company Limited US Patent | Assay Description The 5-HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux ... | US Patent US9346829 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM233392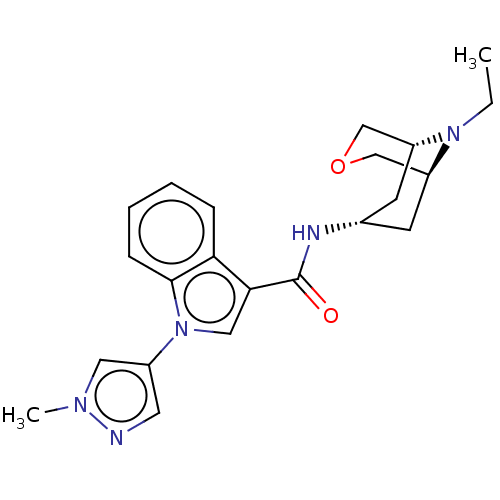 (US9346829, 31) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Phamaceutical Company Limited US Patent | Assay Description The 5-HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux ... | US Patent US9346829 (2016) BindingDB Entry DOI: 10.7270/Q2MK6BRB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||