

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM237158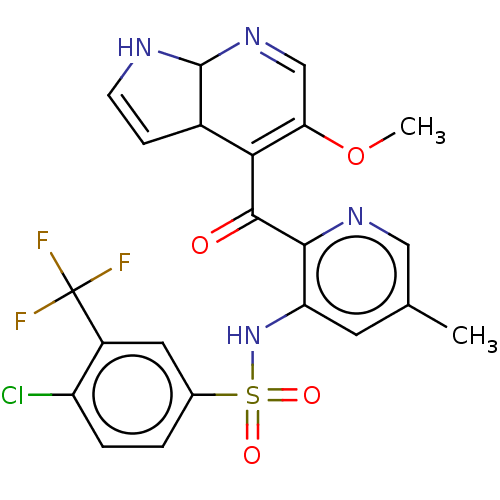 (US9394307, 4-chloro-N-(2-{5-methoxy-1H,3aH,7aH-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
ChemoCentryx, Inc. US Patent | Assay Description The test compounds were incubated with pooled human liver microsomes at 37° C. in the presence of NADPH and appropriate concentrations of substra... | US Patent US9394307 (2016) BindingDB Entry DOI: 10.7270/Q2NZ86JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM237157 (US9394307, 4-chloro-N-(5-methyl-2-{1H-pyrazolo[3,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
ChemoCentryx, Inc. US Patent | Assay Description The test compounds were incubated with pooled human liver microsomes at 37° C. in the presence of NADPH and appropriate concentrations of substra... | US Patent US9394307 (2016) BindingDB Entry DOI: 10.7270/Q2NZ86JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM237159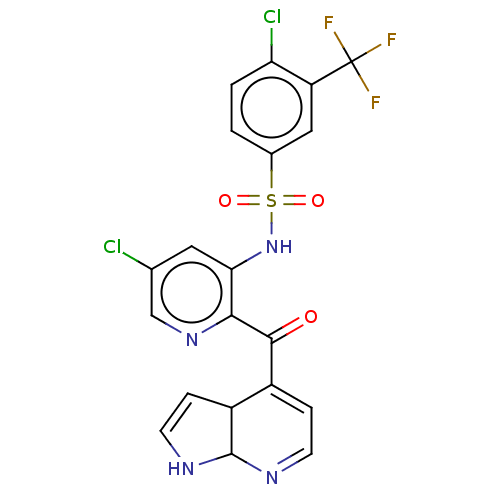 (US9394307, N-(2-{1H,3aH,7aH-pyrrolo[2,3-b]pyridine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
ChemoCentryx, Inc. US Patent | Assay Description The test compounds were incubated with pooled human liver microsomes at 37° C. in the presence of NADPH and appropriate concentrations of substra... | US Patent US9394307 (2016) BindingDB Entry DOI: 10.7270/Q2NZ86JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM237160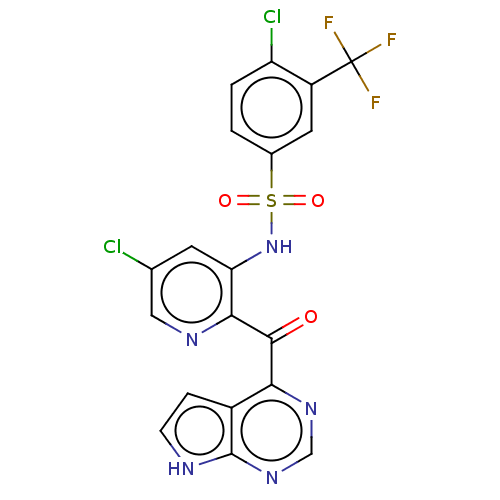 (US9394307, 4-chloro-N-(5-chloro-2-{7H-pyrrolo[2,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
ChemoCentryx, Inc. US Patent | Assay Description The test compounds were incubated with pooled human liver microsomes at 37° C. in the presence of NADPH and appropriate concentrations of substra... | US Patent US9394307 (2016) BindingDB Entry DOI: 10.7270/Q2NZ86JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50398343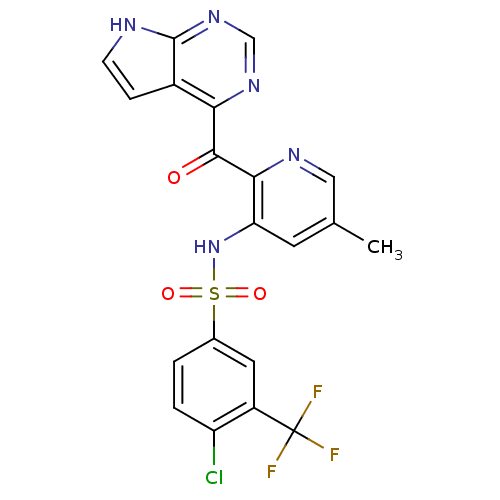 (CHEMBL2178573 | US9394307, 4-chloro-N-(5-methyl-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
ChemoCentryx, Inc. US Patent | Assay Description The test compounds were incubated with pooled human liver microsomes at 37° C. in the presence of NADPH and appropriate concentrations of substra... | US Patent US9394307 (2016) BindingDB Entry DOI: 10.7270/Q2NZ86JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50398343 (CHEMBL2178573 | US9394307, 4-chloro-N-(5-methyl-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
ChemoCentryx, Inc. US Patent | Assay Description The test compounds were incubated with pooled human liver microsomes at 37° C. in the presence of NADPH and appropriate concentrations of substra... | US Patent US9394307 (2016) BindingDB Entry DOI: 10.7270/Q2NZ86JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||