

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242568 (US9422240, 1-378) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242567 (US9422240, 1-377) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242554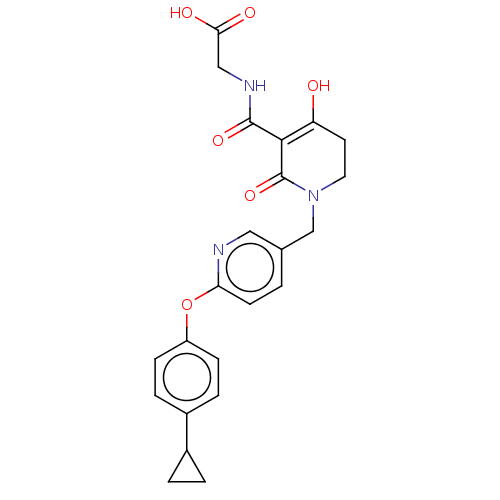 (US9422240, 1-296) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242563 (US9422240, 1-366) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242526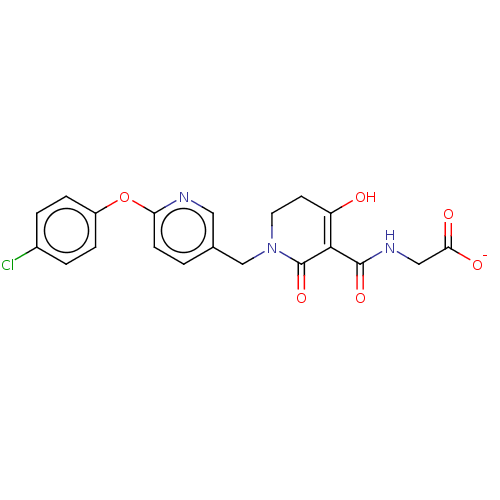 (US9422240, 1-4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242577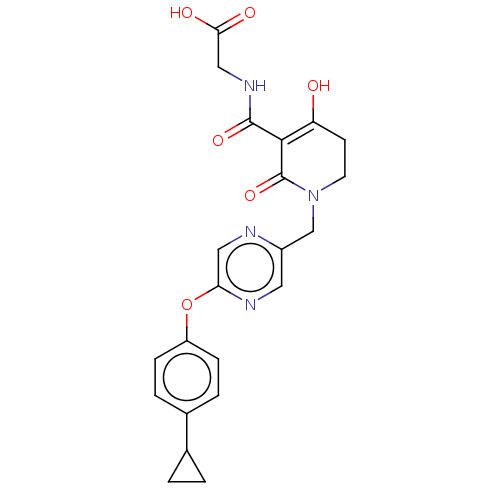 (US9422240, 1-443) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242566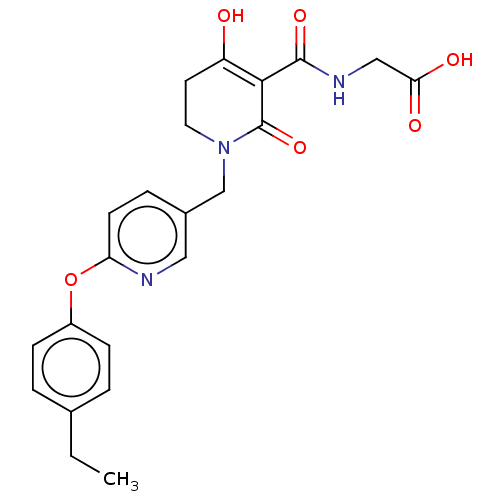 (US9422240, 1-376) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242542 (US9422240, 1-265 | US9422240, 1-355) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242535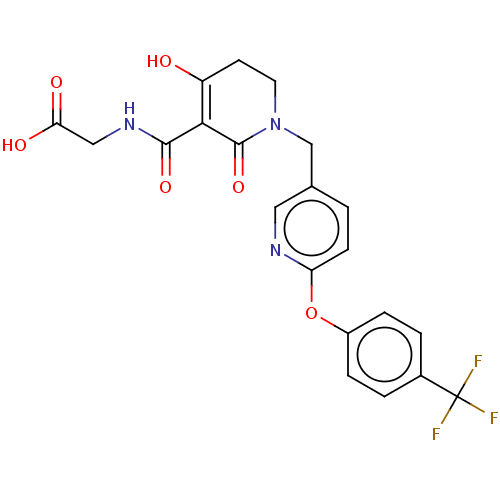 (US9422240, 1-254) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242575 (US9422240, 1-409) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 25 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242571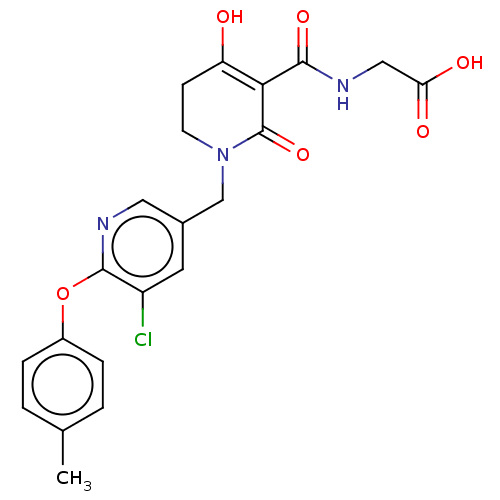 (US9422240, 1-392) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242564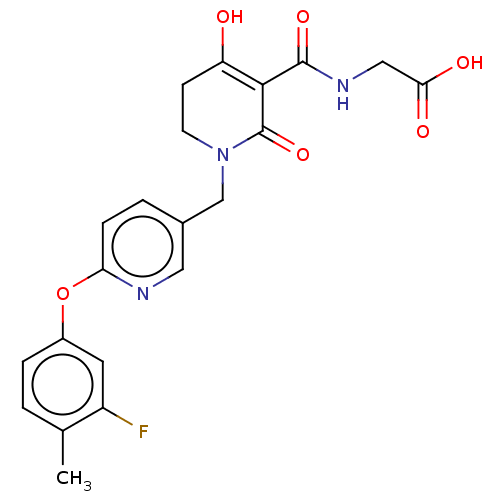 (US9422240, 1-367) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242572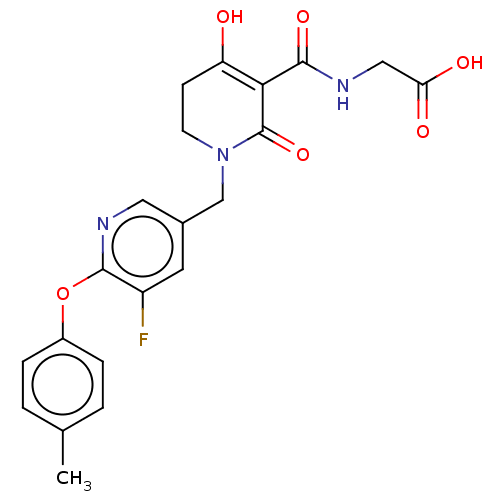 (US9422240, 1-393) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 32 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242576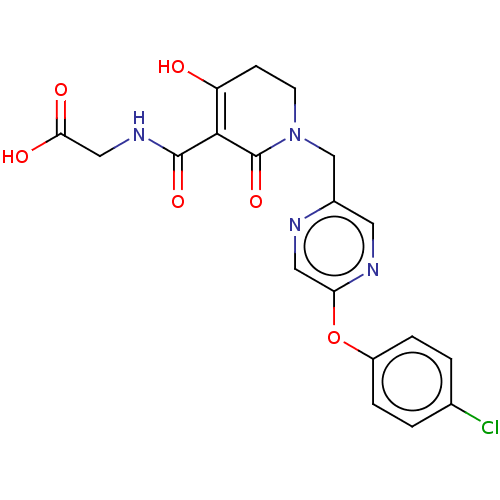 (US9422240, 1-440) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 39 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242528 (US9422240, 1-70) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 39 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242548 (US9422240, 1-284) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242532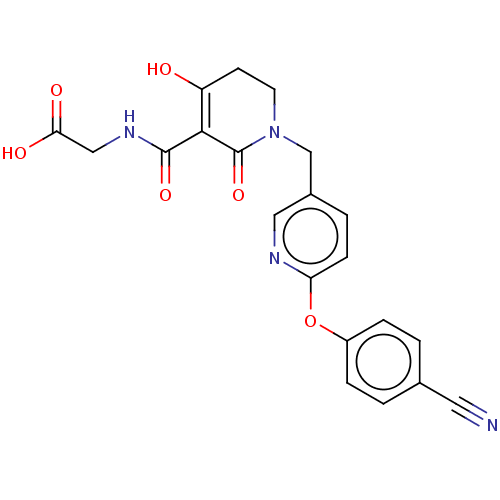 (US9422240, 1-225) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242565 (US9422240, 1-368) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 41 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242558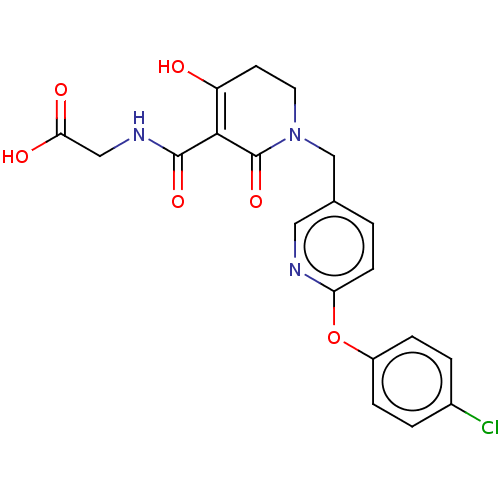 (US9422240, 1-353) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 43 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242549 (US9422240, 1-285) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 43 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242551 (US9422240, 1-287) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 45 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242570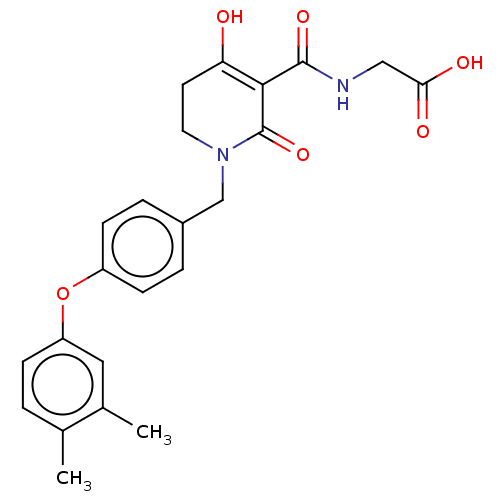 (US9422240, 1-386) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 48 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242531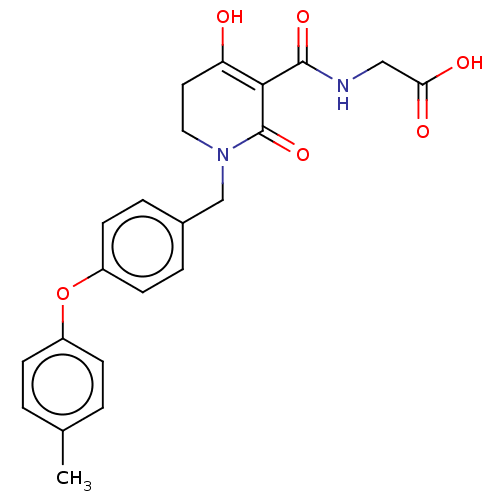 (US9422240, 1-209) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 49 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242542 (US9422240, 1-265 | US9422240, 1-355) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 52 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242569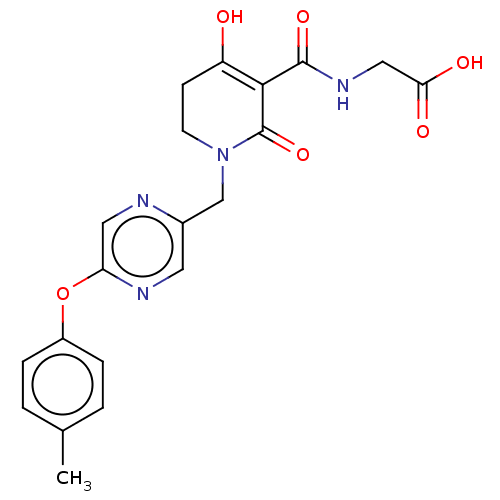 (US9422240, 1-385) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 54 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242562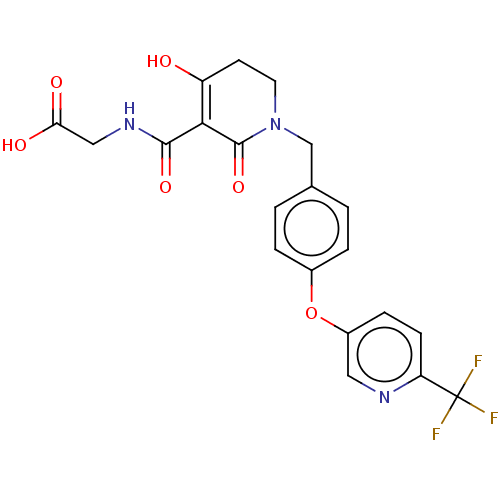 (US9422240, 1-365) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 56 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242573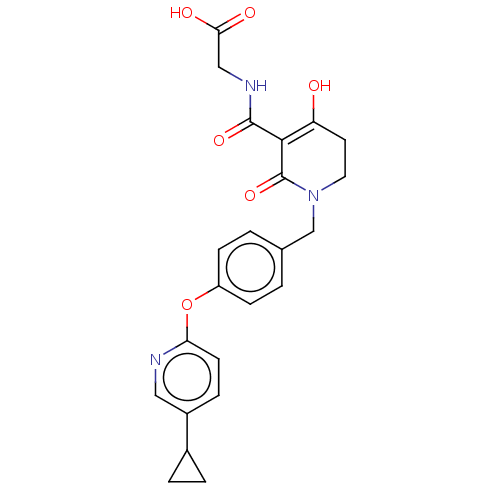 (US9422240, 1-404) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 56 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242530 (US9422240, 1-205) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 59 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242561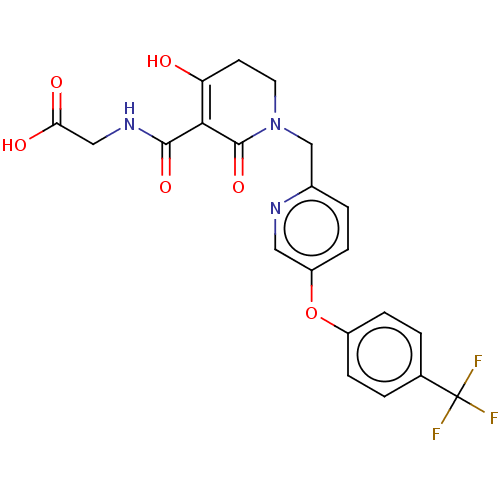 (US9422240, 1-356) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242559 (US9422240, 1-354) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 61 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242550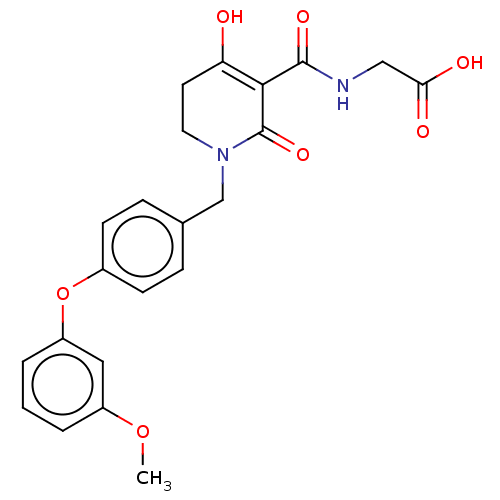 (US9422240, 1-286) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 61 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242539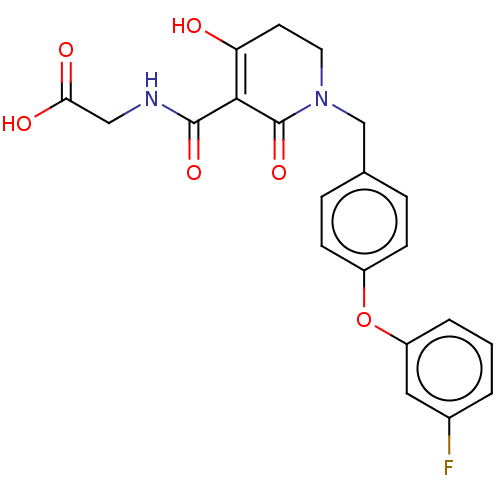 (US9422240, 1-258) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 63 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242547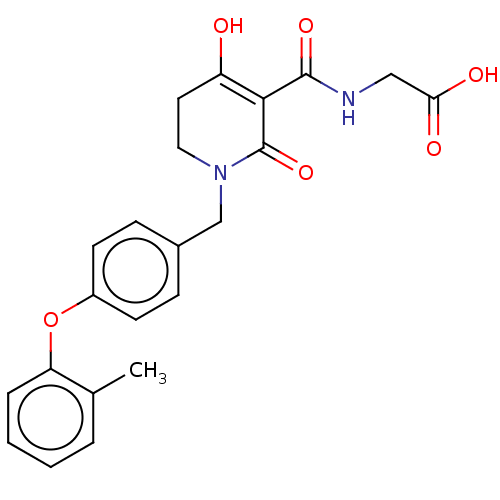 (US9422240, 1-283) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 63 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242537 (US9422240, 1-256) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 66 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242536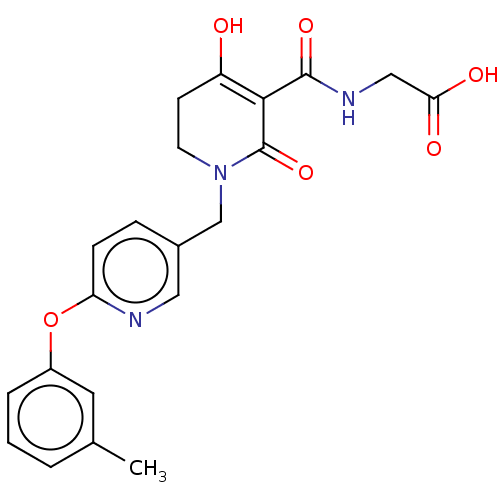 (US9422240, 1-255) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 66 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242553 (US9422240, 1-289) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 68 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242541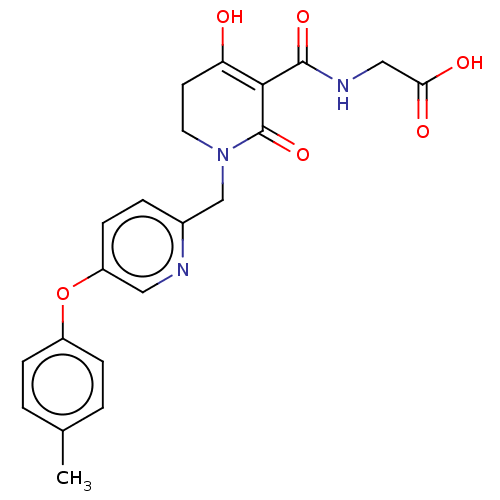 (US9422240, 1-260) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 69 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242574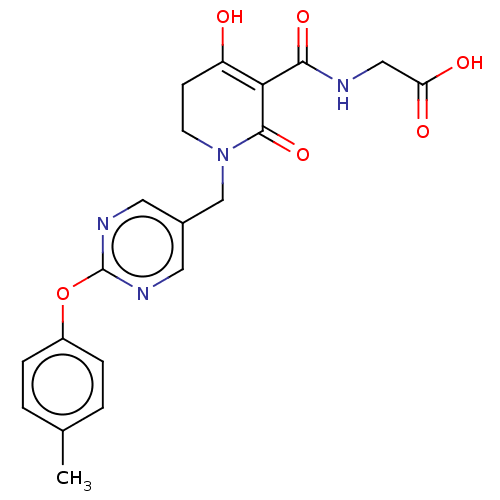 (US9422240, 1-408) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 72 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242538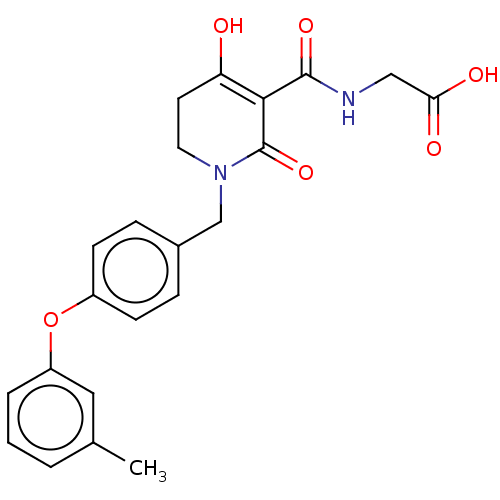 (US9422240, 1-257) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 75 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242546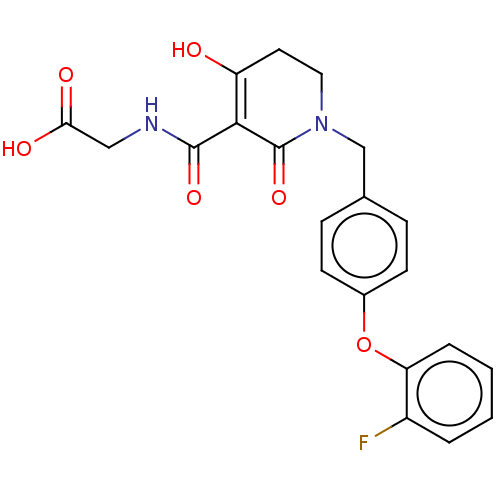 (US9422240, 1-282) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 77 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242556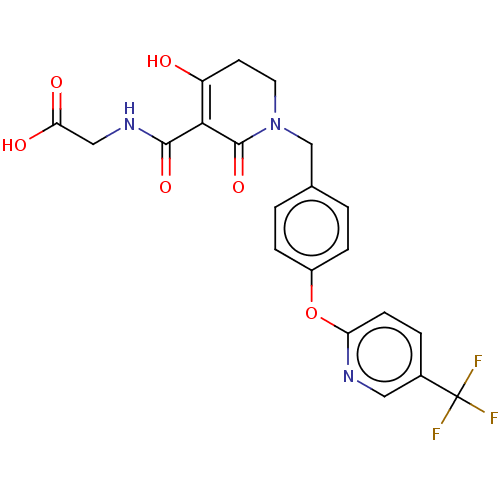 (US9422240, 1-298) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 79 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242534 (US9422240, 1-252) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 87 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242527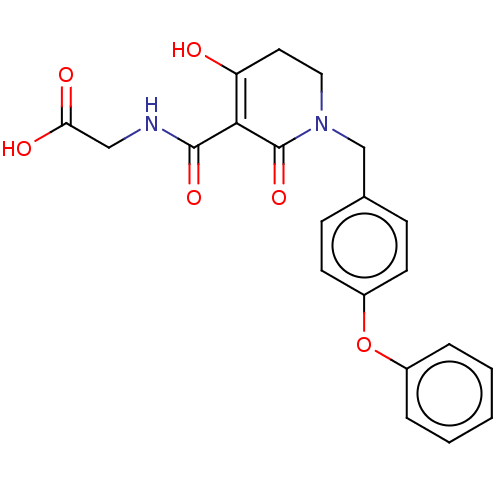 (US9422240, 1-28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 88 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242555 (US9422240, 1-297) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 89 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242557 (US9422240, 1-333) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 92 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242529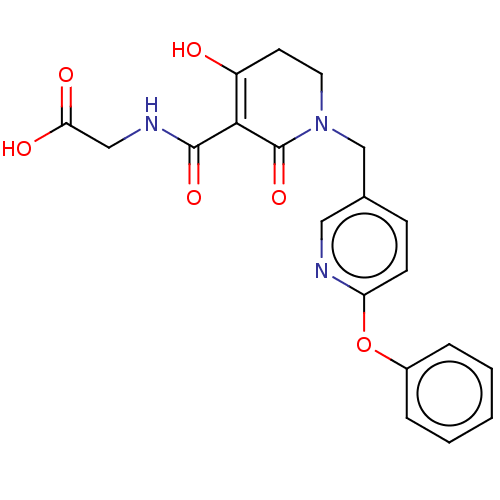 (US9422240, 1-72) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 94 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242540 (US9422240, 1-259) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 96 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242545 (US9422240, 1-275) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 99 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242544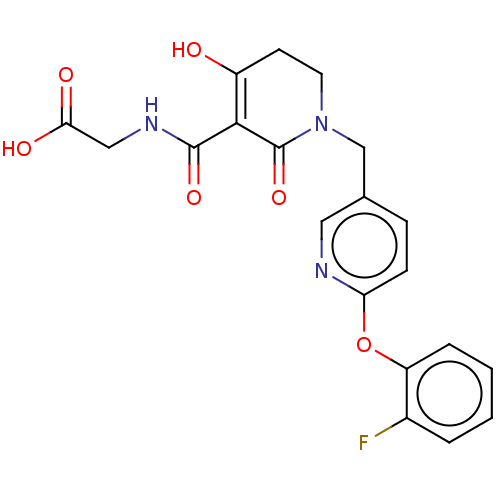 (US9422240, 1-274) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 108 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242543 (US9422240, 1-273) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 112 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242552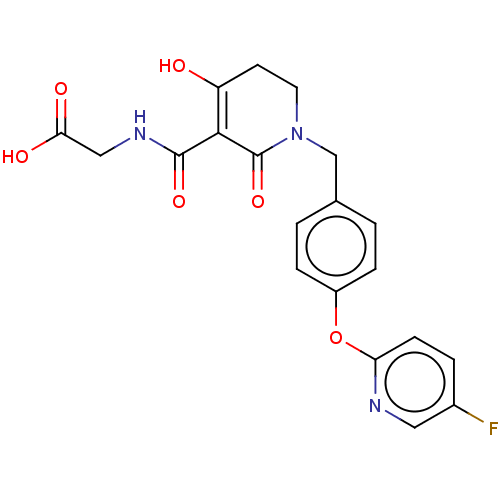 (US9422240, 1-288) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 146 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242533 (US9422240, 1-226) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 409 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||