

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM244799 (US9447055, I) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono S.A. US Patent | Assay Description The potency of inhibition of compound I and compound II on prostaglandin F2α receptor was assessed by analyzing the affinity of these compounds... | US Patent US9447055 (2016) BindingDB Entry DOI: 10.7270/Q2M61J6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM244800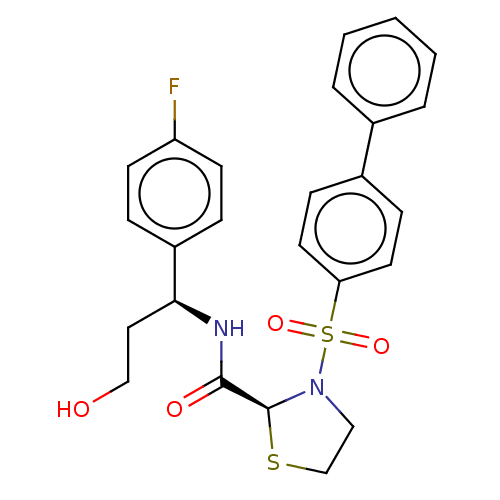 (US9447055, II) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono S.A. US Patent | Assay Description The potency of inhibition of compound I and compound II on prostaglandin F2α receptor was assessed by analyzing the affinity of these compounds... | US Patent US9447055 (2016) BindingDB Entry DOI: 10.7270/Q2M61J6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||