

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256644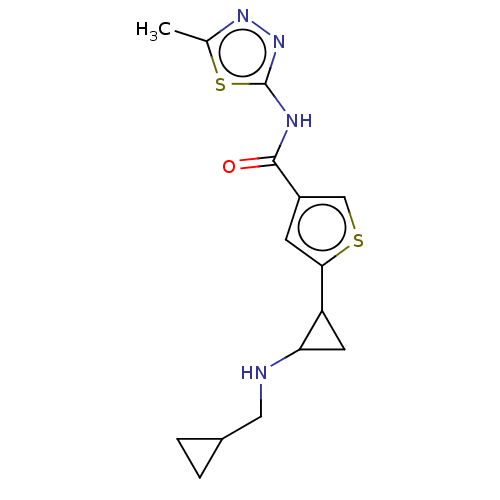 (US10053456, 23 | US10414761, Example 23 | US109682...) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256666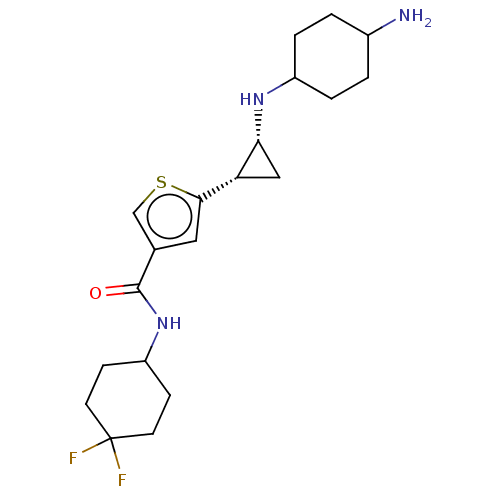 (BDBM256667 | US9487511, 45) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256683 (US10053456, 62 | US10414761, Example 62 | US109682...) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM65037 (BDBM65038 | US9487511, 71) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256669 (US9487511, 48) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256637 (US10053456, 16 | US10414761, Example 16 | US109682...) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256651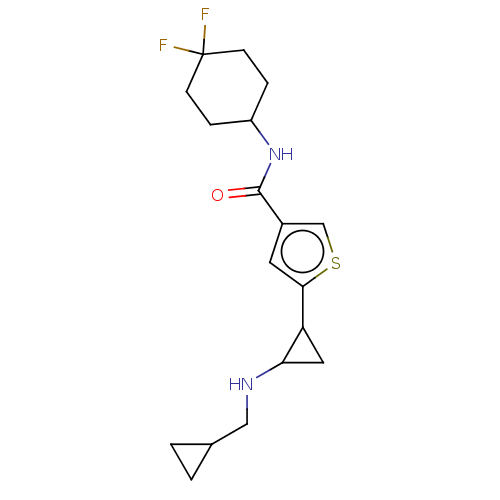 (US10053456, 30 | US10414761, Example 30 | US109682...) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256659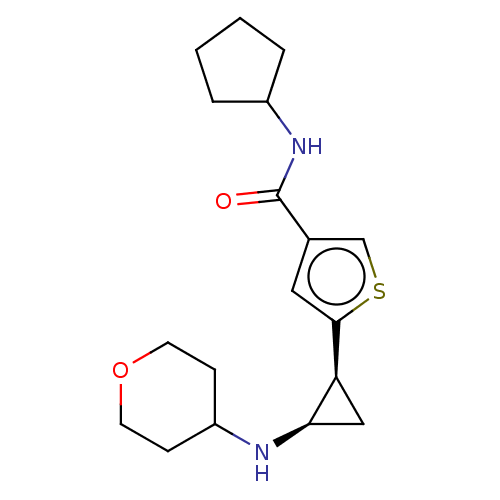 (US9487511, 38) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256704 (US9487511, 86) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256716 (US9487511, 101) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM65037 (BDBM65038 | US9487511, 71) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256729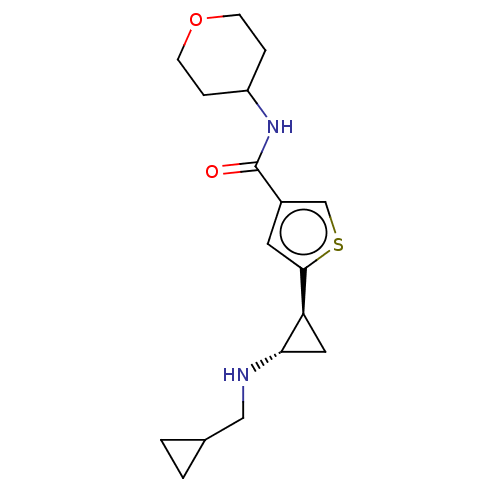 (US10053456, 125 | US10414761, Example 125 | US1096...) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256652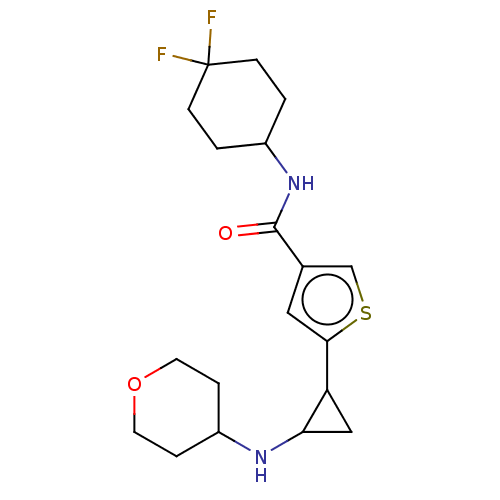 (US10053456, 31 | US10414761, Example 31 | US109682...) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM65037 (BDBM65038 | US9487511, 71) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256641 (US10053456, 20 | US10414761, Example 20 | US109682...) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256710 (US9487511, 92) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM50153591 (CHEMBL3775711 | US10053456, 99 | US10414761, Examp...) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM50153593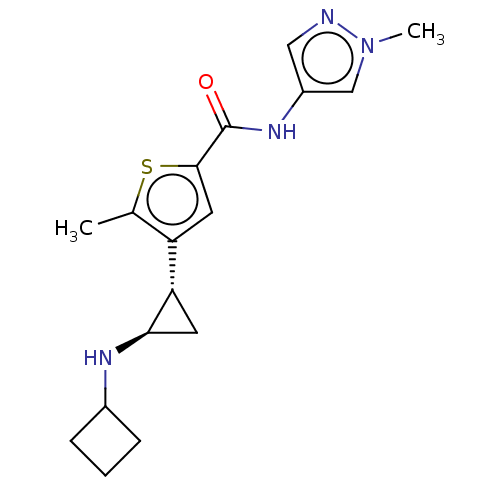 (CHEMBL3775351 | US10053456, 123 | US10414761, Exam...) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256700 (US9487511, 80) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256707 (US10053456, 89 | US10414761, Example 89 | US948751...) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM50153590 (CHEMBL3774809 | US10053456, 97 | US10414761, Examp...) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256653 (US10053456, 32 | US10414761, Example 32 | US109682...) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256654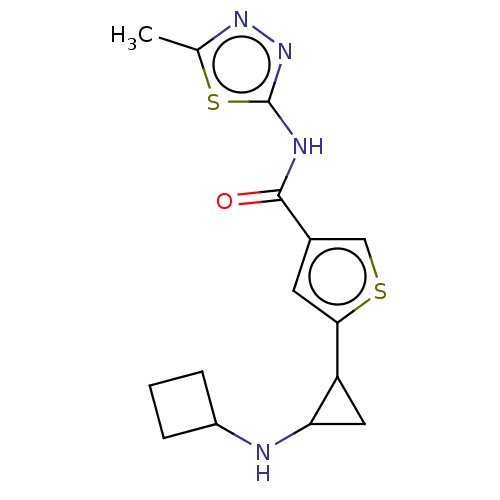 (US10053456, 33 | US10414761, Example 33 | US109682...) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256661 (BDBM256662 | US10053456, 41 | US10414761, Example ...) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256672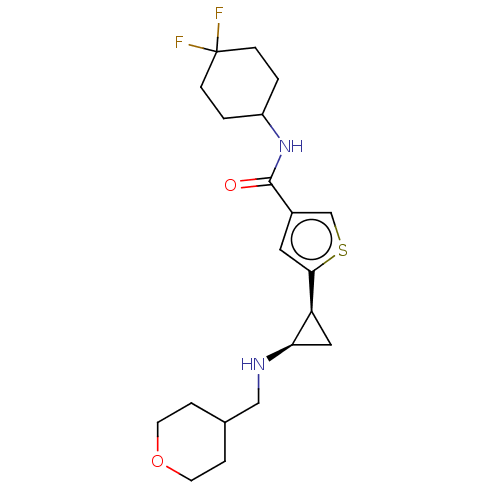 (US9487511, 51) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256686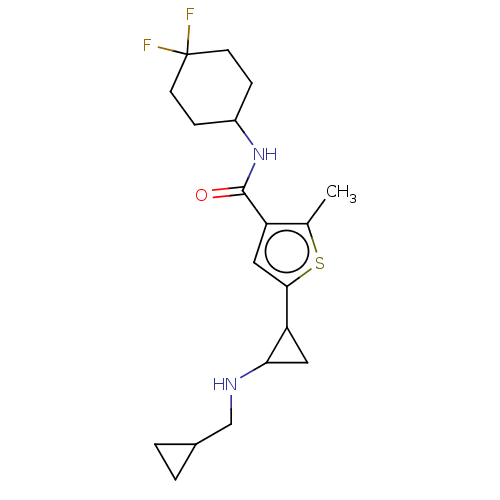 (US10053456, 65 | US10414761, Example 65 | US109682...) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256691 (US10053456, 70 | US10414761, Example 70 | US109682...) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256706 (US10053456, 88 | US10414761, Example 88 | US109682...) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256649 (US10053456, 28 | US10414761, Example 28 | US109682...) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256655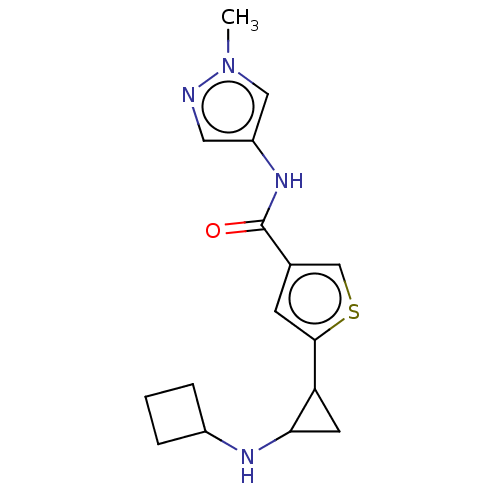 (US10053456, 34 | US10414761, Example 34 | US109682...) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256663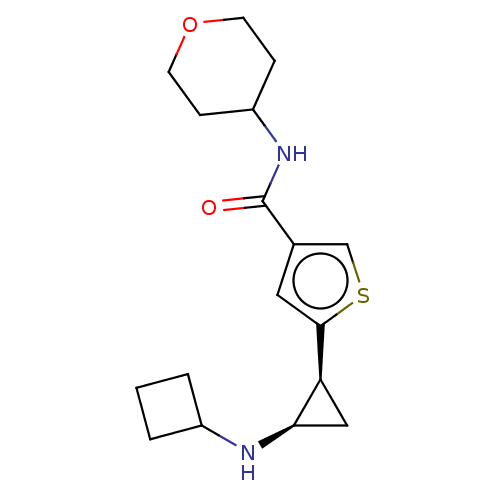 (US9487511, 42) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256671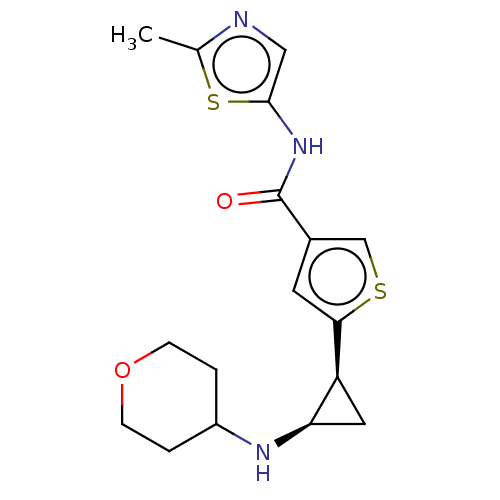 (US9487511, 50) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256676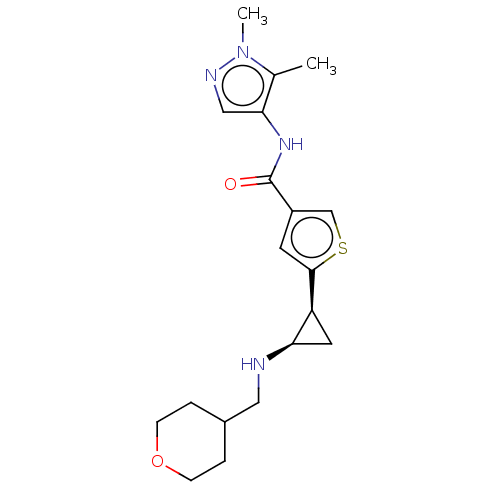 (US9487511, 55) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256692 (US10053456, 72 | US10414761, Example 72 | US109682...) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256701 (US9487511, 81) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM65037 (BDBM65038 | US9487511, 71) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256633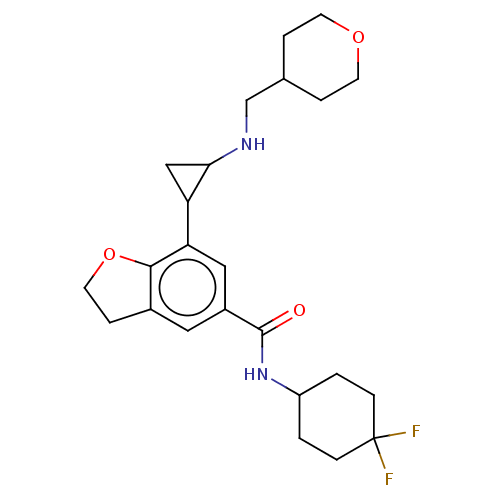 (US10053456, 12 | US10414761, Example 12 | US109682...) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256670 (US9487511, 49) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256675 (US9487511, 54) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256677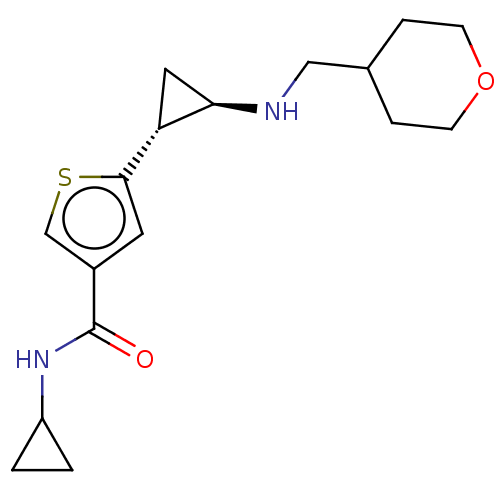 (US10053456, 56 | US10414761, Example 56 | US109682...) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256679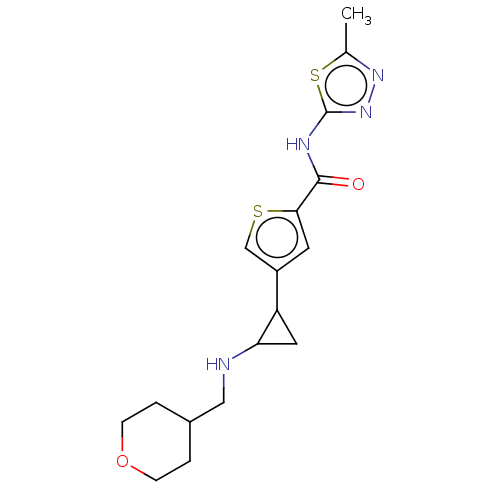 (US10053456, 58 | US10414761, Example 58 | US109682...) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM65037 (BDBM65038 | US9487511, 71) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256724 (US10053456, 111 | US10414761, Example 111 | US1096...) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256725 (US9487511, 112) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256660 (US9487511, 39) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256673 (US9487511, 52) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256645 (US10053456, 24 | US10414761, Example 24 | US109682...) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256668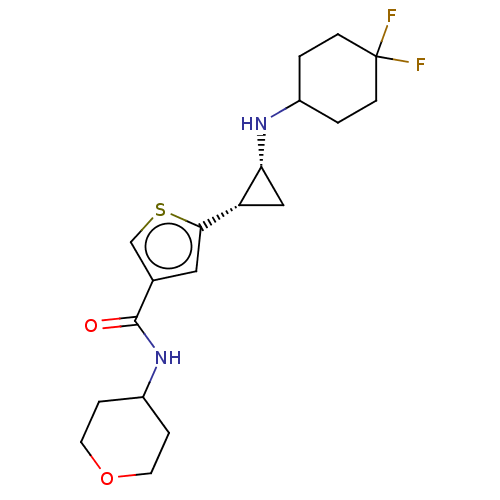 (US9487511, 47) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256674 (US9487511, 53) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P/Lysine-specific histone demethylase 1A [172-833] (Homo sapiens (Human)) | BDBM256688 (US10053456, 67 | US10414761, Example 67 | US109682...) | PDB UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Takeda Pharmaceutical Company Limited US Patent | Assay Description A test compound dissolved in DMSO was added by to a reaction solution (50 mM Tris-HCl (pH 8.0), 0.1% BSA, 1 mM DTT) containing LSD1 enzyme, and the m... | US Patent US9487511 (2016) BindingDB Entry DOI: 10.7270/Q2V123Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 375 total ) | Next | Last >> |