

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM258436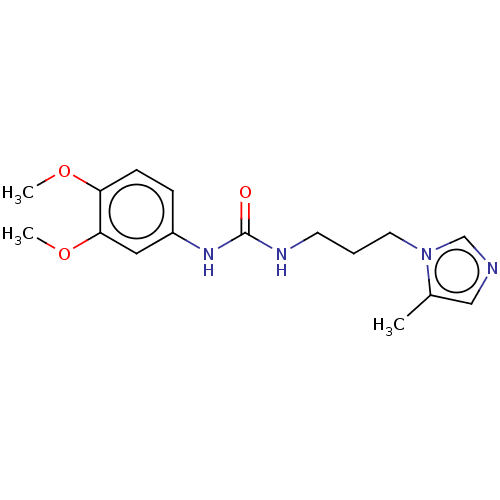 (US9512082, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 31.2 | -43.6 | 192 | n/a | n/a | n/a | n/a | 8.0 | 30 |
PROBIODRUG AG US Patent | Assay Description For inhibitor testing, the sample composition was the same as described below, except of the putative inhibitory compound added. For a rapid test of ... | US Patent US9512082 (2016) BindingDB Entry DOI: 10.7270/Q2CR5S92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM258437 (US9512082, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 60 | -41.9 | 343 | n/a | n/a | n/a | n/a | 8.0 | 30 |
PROBIODRUG AG US Patent | Assay Description For inhibitor testing, the sample composition was the same as described below, except of the putative inhibitory compound added. For a rapid test of ... | US Patent US9512082 (2016) BindingDB Entry DOI: 10.7270/Q2CR5S92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM258442 (US9512082, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 171 | -39.3 | 1.67E+3 | n/a | n/a | n/a | n/a | 8.0 | 30 |
PROBIODRUG AG US Patent | Assay Description For inhibitor testing, the sample composition was the same as described below, except of the putative inhibitory compound added. For a rapid test of ... | US Patent US9512082 (2016) BindingDB Entry DOI: 10.7270/Q2CR5S92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM258441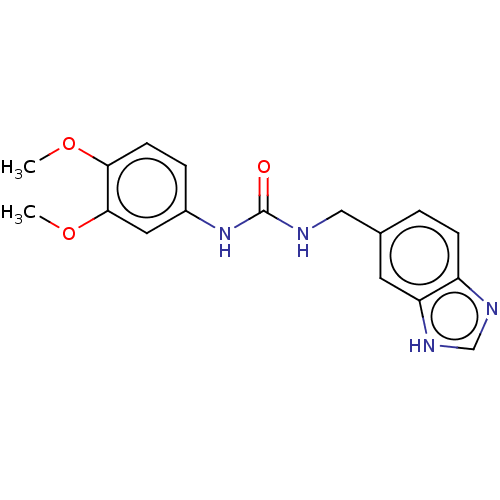 (US9512082, 6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 458 | -36.8 | 4.25E+3 | n/a | n/a | n/a | n/a | 8.0 | 30 |
PROBIODRUG AG US Patent | Assay Description For inhibitor testing, the sample composition was the same as described below, except of the putative inhibitory compound added. For a rapid test of ... | US Patent US9512082 (2016) BindingDB Entry DOI: 10.7270/Q2CR5S92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM258438 (US9512082, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.77E+3 | -33.4 | 1.40E+4 | n/a | n/a | n/a | n/a | 8.0 | 30 |
PROBIODRUG AG US Patent | Assay Description For inhibitor testing, the sample composition was the same as described below, except of the putative inhibitory compound added. For a rapid test of ... | US Patent US9512082 (2016) BindingDB Entry DOI: 10.7270/Q2CR5S92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM258440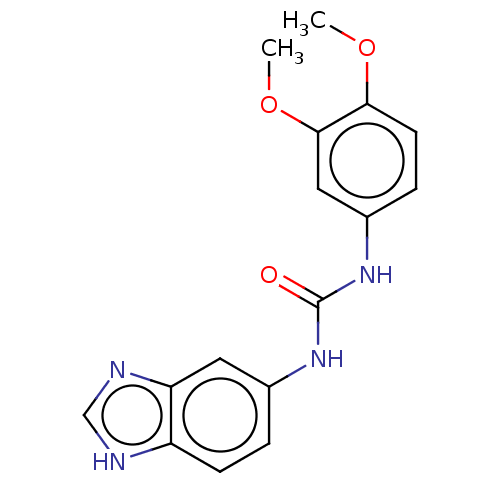 (US9512082, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.98E+3 | -32.1 | 2.62E+4 | n/a | n/a | n/a | n/a | 8.0 | 30 |
PROBIODRUG AG US Patent | Assay Description For inhibitor testing, the sample composition was the same as described below, except of the putative inhibitory compound added. For a rapid test of ... | US Patent US9512082 (2016) BindingDB Entry DOI: 10.7270/Q2CR5S92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM258439 (US9512082, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.82E+3 | n/a | n/a | n/a | n/a | 8.0 | 30 |
PROBIODRUG AG US Patent | Assay Description For inhibitor testing, the sample composition was the same as described below, except of the putative inhibitory compound added. For a rapid test of ... | US Patent US9512082 (2016) BindingDB Entry DOI: 10.7270/Q2CR5S92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||