 Found 9 hits Enzyme Inhibition Constant Data
Found 9 hits Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Farnesyl pyrophosphate synthase
(348/348 = 100%)†
(Homo sapiens (Human)) | BDBM12581
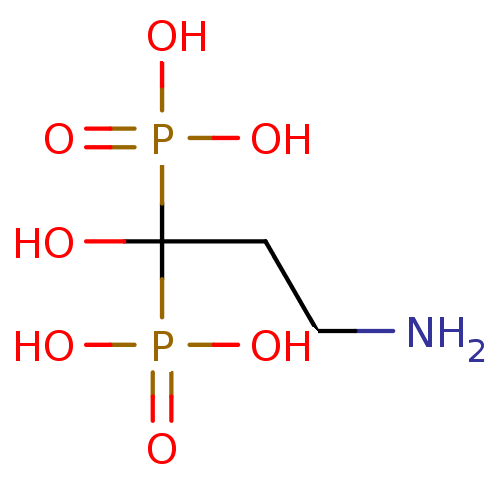
((3-amino-1-hydroxy-1-phosphonopropyl)phosphonic ac...)Show InChI InChI=1S/C3H11NO7P2/c4-2-1-3(5,12(6,7)8)13(9,10)11/h5H,1-2,4H2,(H2,6,7,8)(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 55.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(348/348 = 100%)†
(Homo sapiens (Human)) | BDBM12581

((3-amino-1-hydroxy-1-phosphonopropyl)phosphonic ac...)Show InChI InChI=1S/C3H11NO7P2/c4-2-1-3(5,12(6,7)8)13(9,10)11/h5H,1-2,4H2,(H2,6,7,8)(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand |
J Med Chem 46: 5171-83 (2003)
Article DOI: 10.1021/jm0302344
BindingDB Entry DOI: 10.7270/Q24M93Z8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(348/348 = 100%)†
(Homo sapiens (Human)) | BDBM12581

((3-amino-1-hydroxy-1-phosphonopropyl)phosphonic ac...)Show InChI InChI=1S/C3H11NO7P2/c4-2-1-3(5,12(6,7)8)13(9,10)11/h5H,1-2,4H2,(H2,6,7,8)(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 331 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(348/348 = 100%)†
(Homo sapiens (Human)) | BDBM12581

((3-amino-1-hydroxy-1-phosphonopropyl)phosphonic ac...)Show InChI InChI=1S/C3H11NO7P2/c4-2-1-3(5,12(6,7)8)13(9,10)11/h5H,1-2,4H2,(H2,6,7,8)(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibitory activity against the human recombinant FPPSase(Farnesyl diphosphate) enzyme |
J Med Chem 45: 2185-96 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0MN1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(348/348 = 100%)†
(Homo sapiens (Human)) | BDBM12581

((3-amino-1-hydroxy-1-phosphonopropyl)phosphonic ac...)Show InChI InChI=1S/C3H11NO7P2/c4-2-1-3(5,12(6,7)8)13(9,10)11/h5H,1-2,4H2,(H2,6,7,8)(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human FPPS expressed in Escherichia coli by scintillation counting |
J Med Chem 54: 5955-80 (2011)
Article DOI: 10.1021/jm200587f
BindingDB Entry DOI: 10.7270/Q2PV6MGF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(348/348 = 100%)†
(Homo sapiens (Human)) | BDBM12581

((3-amino-1-hydroxy-1-phosphonopropyl)phosphonic ac...)Show InChI InChI=1S/C3H11NO7P2/c4-2-1-3(5,12(6,7)8)13(9,10)11/h5H,1-2,4H2,(H2,6,7,8)(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 353 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 after 10 mins |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(348/348 = 100%)†
(Homo sapiens (Human)) | BDBM12581

((3-amino-1-hydroxy-1-phosphonopropyl)phosphonic ac...)Show InChI InChI=1S/C3H11NO7P2/c4-2-1-3(5,12(6,7)8)13(9,10)11/h5H,1-2,4H2,(H2,6,7,8)(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibitory activity against farnesyl Pyrophosphate Synthase expressed as #NAME? (M) |
J Med Chem 46: 5171-83 (2003)
Article DOI: 10.1021/jm0302344
BindingDB Entry DOI: 10.7270/Q24M93Z8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(348/348 = 100%)†
(Homo sapiens (Human)) | BDBM12581

((3-amino-1-hydroxy-1-phosphonopropyl)phosphonic ac...)Show InChI InChI=1S/C3H11NO7P2/c4-2-1-3(5,12(6,7)8)13(9,10)11/h5H,1-2,4H2,(H2,6,7,8)(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibitory activity against farnesyl Pyrophosphate Synthase was determined |
J Med Chem 46: 5171-83 (2003)
Article DOI: 10.1021/jm0302344
BindingDB Entry DOI: 10.7270/Q24M93Z8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(348/348 = 100%)†
(Homo sapiens (Human)) | BDBM12581

((3-amino-1-hydroxy-1-phosphonopropyl)phosphonic ac...)Show InChI InChI=1S/C3H11NO7P2/c4-2-1-3(5,12(6,7)8)13(9,10)11/h5H,1-2,4H2,(H2,6,7,8)(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS expressed in Escherichia coli BL21 |
J Med Chem 51: 2187-95 (2008)
Article DOI: 10.1021/jm7015733
BindingDB Entry DOI: 10.7270/Q2W95B18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data