 Found 5 hits Enzyme Inhibition Constant Data
Found 5 hits Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
MAP kinase-activated protein kinase 2
(327/327 = 100%)†
(Homo sapiens (Human)) | BDBM30192
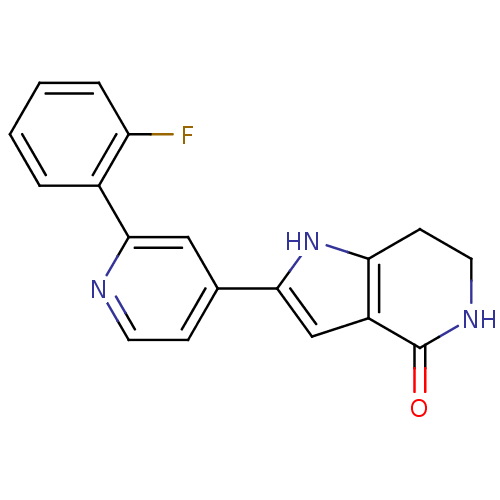
(Pyrrolopyridine, 23)Show InChI InChI=1S/C18H14FN3O/c19-14-4-2-1-3-12(14)17-9-11(5-7-20-17)16-10-13-15(22-16)6-8-21-18(13)23/h1-5,7,9-10,22H,6,8H2,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Pfizer
| Assay Description
The phosphorylation of HSP27 peptide by MAPKAPK2 was measured using an anion exchange resin capture assay method. The reaction was carried out in rea... |
J Med Chem 50: 2647-54 (2007)
Article DOI: 10.1021/jm0611004
BindingDB Entry DOI: 10.7270/Q2794313 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
MAP kinase-activated protein kinase 2
(327/327 = 100%)†
(Homo sapiens (Human)) | BDBM30192

(Pyrrolopyridine, 23)Show InChI InChI=1S/C18H14FN3O/c19-14-4-2-1-3-12(14)17-9-11(5-7-20-17)16-10-13-15(22-16)6-8-21-18(13)23/h1-5,7,9-10,22H,6,8H2,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
MAP kinase-activated protein kinase 2
(327/327 = 100%)†
(Homo sapiens (Human)) | BDBM30192

(Pyrrolopyridine, 23)Show InChI InChI=1S/C18H14FN3O/c19-14-4-2-1-3-12(14)17-9-11(5-7-20-17)16-10-13-15(22-16)6-8-21-18(13)23/h1-5,7,9-10,22H,6,8H2,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of MK2 mediated anisomycin-stimulated hsp27 phosphorylation in human THP-1 cells by fluorometric analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
MAP kinase-activated protein kinase 2
(327/327 = 100%)†
(Homo sapiens (Human)) | BDBM30192

(Pyrrolopyridine, 23)Show InChI InChI=1S/C18H14FN3O/c19-14-4-2-1-3-12(14)17-9-11(5-7-20-17)16-10-13-15(22-16)6-8-21-18(13)23/h1-5,7,9-10,22H,6,8H2,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 in LPS-stimulated human THP1 cells assessed as inhibition of Hsp27 phosphorylation pretreated 60 mins before LPS challenge measured... |
Bioorg Med Chem Lett 21: 3823-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.016
BindingDB Entry DOI: 10.7270/Q2FT8MCB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
MAP kinase-activated protein kinase 2
(327/327 = 100%)†
(Homo sapiens (Human)) | BDBM30192

(Pyrrolopyridine, 23)Show InChI InChI=1S/C18H14FN3O/c19-14-4-2-1-3-12(14)17-9-11(5-7-20-17)16-10-13-15(22-16)6-8-21-18(13)23/h1-5,7,9-10,22H,6,8H2,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 372 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 pretreated for 30 mins before fluorescein labeled substrate peptide addition measured after 2 hrs by IMAP assay |
Bioorg Med Chem Lett 21: 3823-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.016
BindingDB Entry DOI: 10.7270/Q2FT8MCB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data