

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methionine aminopeptidase (263/263 = 100%)† (Escherichia coli (strain K12)) | BDBM50175432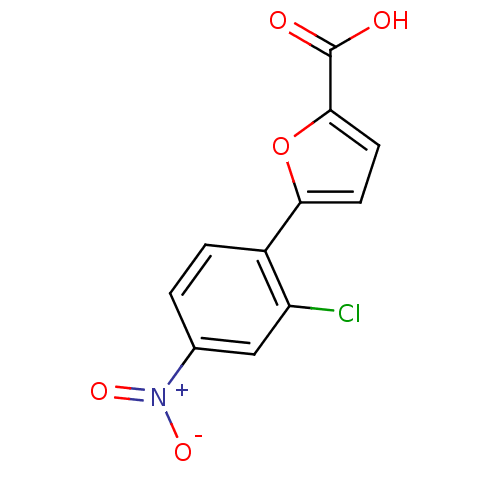 (5-(2-CHLORO-4-NITROPHENYL)-2-FUROIC ACID | 5-(2-ch...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem | DrugBank MMDB PDB Article PubMed | n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Mn(II) form of Escherichia coli MetAP | Bioorg Med Chem Lett 15: 5386-91 (2005) Article DOI: 10.1016/j.bmcl.2005.09.019 BindingDB Entry DOI: 10.7270/Q2HH6JNV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methionine aminopeptidase (263/263 = 100%)† (Escherichia coli (strain K12)) | BDBM50175432 (5-(2-CHLORO-4-NITROPHENYL)-2-FUROIC ACID | 5-(2-ch...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem | DrugBank MMDB PDB Article PubMed | n/a | n/a | 3.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Co(II) form of Escherichia coli MetAP | Bioorg Med Chem Lett 15: 5386-91 (2005) Article DOI: 10.1016/j.bmcl.2005.09.019 BindingDB Entry DOI: 10.7270/Q2HH6JNV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methionine aminopeptidase (263/263 = 100%)† (Escherichia coli (strain K12)) | BDBM50175432 (5-(2-CHLORO-4-NITROPHENYL)-2-FUROIC ACID | 5-(2-ch...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem | DrugBank MMDB PDB Article PubMed | n/a | n/a | 4.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Ni(II) form of Escherichia coli MetAP | Bioorg Med Chem Lett 15: 5386-91 (2005) Article DOI: 10.1016/j.bmcl.2005.09.019 BindingDB Entry DOI: 10.7270/Q2HH6JNV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methionine aminopeptidase (263/263 = 100%)† (Escherichia coli (strain K12)) | BDBM50175432 (5-(2-CHLORO-4-NITROPHENYL)-2-FUROIC ACID | 5-(2-ch...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem | DrugBank MMDB PDB Article PubMed | n/a | n/a | 4.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against Fe(II) form of Escherichia coli MetAP | Bioorg Med Chem Lett 15: 5386-91 (2005) Article DOI: 10.1016/j.bmcl.2005.09.019 BindingDB Entry DOI: 10.7270/Q2HH6JNV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||